Modification of Catalase by Chondroitin Sulfate
A. V. Maksimenko1,2 and E. G. Tischenko1
1Institute of Experimental Cardiology, Russian Cardiology Research Center, ul. 3-ya Cherepkovskaya 15a, Moscow, 121552 Russia; fax: (095) 415-2962; E-mail: csc@adonis.ias.msk.su2To whom correspondence should be addressed.
Submitted July 2, 1997; revision submitted July 16, 1997.
Catalase was chemically modified by sodium chondroitin sulfate using the benzoquinone binding method. Thus, 40-42% of the catalase preparation was modified. Treatment of catalase and superoxide dismutase with benzoquinone-activated chondroitin sulfate results in a bienzymic conjugate with electrophoretically heterogenous composition. The yield of the products and their residual catalytic activity indicate that the method can be used for the preparation of modified catalase and the bienzymic conjugate to study their efficiency in vivo.
KEY WORDS: catalase, chondroitin sulfate, superoxide dismutase, chemical modification, covalent conjugation.
Unbalanced increase in the flux of free radicals in the body promotes the development of various lesions. In particular, spontaneous platelet aggregation is enhanced [1] thus causing re-occlusions, generation of aggressive hydroxyl radical, and accumulation of hydrogen peroxide [2]. Hydrogen peroxide promotes platelet aggregation at subthreshold concentrations of inducers (arachidonic acid, collagen) [3] and increases the probability of unfavorable thrombotic effects. To prevent this, antioxidant enzymes but not systemic antithrombin agents should be used because the enzymes suppress the formation of free radicals [2]. Superoxide dismutase (SOD) and catalase (CAT) are promising representatives of such enzyme preparations [4-6].
CAT is efficient during oxidative stress [7]. For example, the protective function of CAT in erythrocytes includes removal of hydrogen peroxide, whereas the effects of glutathione peroxidase are associated with the removal of organic peroxides [8]. An insignificant role of endogenous CAT in intracellular protection of rat gastric mucosa cultured cells from the effect of exogenous hydrogen peroxide [9] may be due to the absence of CAT on the cell surface. In would be useful to test the protective effects of native and modified CAT.
Polyethylene glycol-modified CAT (see references in [4]) and aldehyde dextran-modified CAT [10] has been used in biomedical studies. Such treatment enhanced the therapeutic effects of CAT due to its modification and coupled functioning with SOD at the damaged site. An additional effect of CAT to the action of SOD within the bienzymic conjugate prepared by coupling the enzymes with aldehyde dextran [10] or glutaric aldehyde [11] was manifested during ischemia/reperfusion of the isolated rat heart [11] and in the rat silicosis model [10]. However, cross-linking of SOD and CAT by bifunctional reagents was not shown to increase the sorption of the conjugates on the cell surface [10, 11]. This can be achieved by chemical modification of the enzymes by a glycosaminoglycan, chondroitin sulfate (CS), which is a component of the cell wall glycocalyx [12].
In the present work, we synthesized CS-modified CAT preparations (CAT--CS and bienzymic conjugate of CAT and SOD) by the benzoquinone binding method that was used for SOD modification [12] and characterized the preparations.
MATERIALS AND METHODS
CAT from bovine liver (specific activity 11,000 U/mg protein) and chondroitin-4-sulfate A (molecular weight 150 kD) from whale sinew were from Sigma (USA). Cu,Zn-SOD was isolated from rat liver (specific activity 1600 U/mg protein) [12]. Benzoquinone, dimethylformamide, and xanthine were from Sigma; xanthine oxidase was from Calbiochem (USA); hydrogen peroxide was from Merck (Germany); nitrotetrazolium blue was from Reanal (Hungary); Sephadex G-25 and Sephacryl S-300 were from Pharmacia (Sweden). All other reagents were of analytical grade (Reakhim, Russia).
Protein content was assayed by the Bradford method [13]. CAT activity was assayed spectrophotometrically by the decrease in optical density (decomposition of hydrogen peroxide) at 240 nm (pH 7.0, room temperature); SOD activity was assayed by inhibition of nitrotetrazolium blue reduction in the xanthine/xanthine oxidase system (pH 7.8) [12, 14]. One unit of CAT activity corresponds to the amount of the enzyme required for the decomposition of 1 µM hydrogen peroxide (at initial concentration 20 mM) per min at 25°C (pH 7.0). One unit of SOD activity corresponds to the amount of the enzyme required for 50% suppression of nitrotetrazolium blue reduction under the experimental conditions [12, 14].
CAT modification by CS included several stages. First, 100 mg of benzoquinone dissolved in 3 ml of dimethylformamide were added to 100 mg of CS dissolved in 6 ml of 0.02 M sodium phosphate buffer containing 150 mM NaCl, pH 6.0 (PBS). The mixture was incubated for 1.5 h at room temperature in the dark. Second, benzoquinone-activated CS was purified. Activated CS was separated from excess benzoquinone on a Sephadex G-25 column (40 ml bed volume) eluted with 0.02 M PBS, pH 6.0. Third, CAT was coupled to activated CS. CAT (32 mg protein) was dissolved in 12 ml of the buffer solution containing 100 mg activated CS; the pH of the mixture was adjusted to 8.5 with 1 M Na2CO3 and the solution was incubated for 20 h at room temperature in the dark. CAT and CS concentrations in the incubation medium were 11 and 55 µM, respectively.
The bienzymic conjugate of CAT and SOD was prepared using activated CS synthesized and purified as described above. SOD (7.5 mg protein) was added to 18 ml of the buffered solution of activated CS (150 mg) and the pH was adjusted to 8.7-9.0 with 1 M Na2CO3; the mixture was incubated for 1.5 h at room temperature in the dark. Then, CAT (20.6 mg protein) was added to the mixture and the pH was adjusted to 8.7-9.0; the solution was incubated for 20 h at room temperature in the dark. CAT, SOD, and CS concentrations in the incubation medium were 5.7, 15.6, and 67 µM, respectively.
Isolation of modified CAT was performed by gel filtration through a Sephacryl S-300 column (40 ml bed volume, fractions of 1.0 ml) equilibrated with 0.05 M PBS, pH 7.5. For large-scale production of the preparations, the incubation medium of chromatographically isolated protein fraction (they yielded similar final products) was filtered through an XM-300 membrane and washed with 0.025 M PBS at pH 7.5. The membrane was washed until the change in optical density at 280 nm of the wash-through was less than 3% of the initial. The isolated preparations were lyophilized.
Electrophoresis of the final and initial preparations was performed through 5-20% polyacrylamide gel in the presence of SDS (Sigma) [15], and the gels were scanned using a 2202 Ultrascan laser densitometer (LKB, Sweden).
RESULTS AND DISCUSSION
To modify such a large protein as CAT (molecular weight 240-250 kD) composed of four identical subunits, a carrier/coupling agent with molecular weight 150 kD should be optimally used [14]; this is why we have chosen the CS preparation. The benzoquinone binding method, similar to other methods of coupling to CAT [10, 11, 14], involves the surface amino groups of the protein. Such treatment should preserve significant residual catalytic activity of the modified enzyme [4, 10, 11].
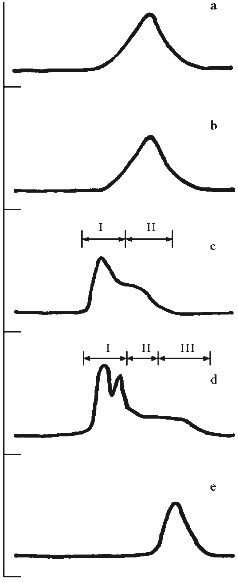
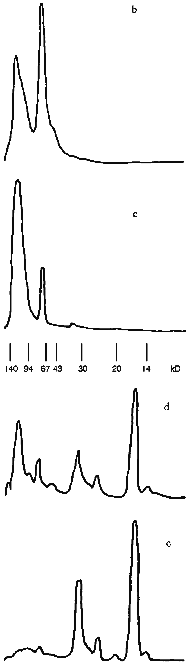
Profiles of gel filtration of CAT preparations (Fig. 1) indicate that the position of the absorption maximum of native CAT (Fig. 1a) does not differ significantly (and profiles of catalytic activity; data not shown) from the position of the peak of the mixture of CAT and CS (Fig. 1b), whereas the treatment of CAT with benzoquinone-activated CS significantly changes the shape of the profile and the position of the peak (Fig. 1c). Separation of the fractions of this profile into volumes I and II (as shown in Fig. 1c) indicates that protein contents in these volumes were 61.3 and 38.7% and CAT activities were 60.2 and 39.8% of the total, respectively. Usually, the fraction of CS-bound CAT after ultrafiltration was 52-60% and residual specific activity after lyophilization was 33-42% of the initial. According to densitometry of electrophoretically separated CAT preparations (Fig. 2, b and c) and comparison of native CAT (Fig. 2a, lane 3) with activated CS-treated CAT (Fig. 2a, lane 2), enzyme modification was 40-42% (according to comparison of peak areas of the densitograms in Fig. 2, b and c). The extent of the modification is sufficient to conclude that at least one modified CAT subunit was included into the catalytically active enzyme tetramer. The data suggest that the efficiency of the derivatives be tested in vivo.
Fig. 1. Elution profiles of CAT and SOD preparations on a Sephacryl S-300 column. Abscissa, fractions; ordinate, absorption at 280 nm. a) Sample of native CAT; b) mixture of CAT and CS; c) modified CAT (CAT--CS conjugate); arrows indicate fractions pooled into volumes I and II; d) bienzymic derivative of CAT and SOD treated with activated CS; arrows indicate fractions pooled into volumes I, II, and III; e) native SOD.
Change in the shape of the gel filtration profile and position of the peaks is characteristic for the bienzymic derivative of CAT and SOD (Fig. 1d). Due to stretching, the profile was separated into three volumes after the fractions indicated in Fig. 1d were pooled. Protein contents in the volumes I, II, and III were 57.5, 27.4, and 15.1%; CAT activities were 49.4, 40.3, and 10.3%; SOD activities were 43.7, 33.4, and 22.9% of the total, respectively. Thus, all CAT and SOD at various ratios form complexes with each other after treatment with activated CS (compare with position of native SOD in Fig. 1e). The mixture of CAT, SOD, and CS does not result in a similar pattern (data not shown). It is important that preparation of the bienzymic conjugate of CAT and SOD by other methods also results in the stretching of the gel filtration profile [10, 11]. However, electrophoretic separation of the derivatives was not performed. Electrophoretic analysis of the bienzymic conjugate indicates that it includes (Fig. 2a, lane 1) native and modified CAT subunits (Fig. 2a, lanes 2 and 3; Fig. 2, b-d), native and modified SOD subunits (Fig. 2a, lanes 6 and 7; Fig. 2, d and e), and other possible oligomeric protein derivatives (Figs. 2a (lane 1) and 2d). The data do not allow more firm conclusions on the organization of the conjugate composition. Comparative study of the efficiency of various preparations of CAT and SOD in vivo should clarify this point. After ultrafiltration and lyophilization of the conjugate, the yield of CAT specific activity is 40-45% and the yield of SOD activity is 65-68% of the initial. Thus, large scale batches of the conjugate can be produced to test its effects in the model of arterial lesions in rats. This biomedical study is the goal of the next stage of our work.Fig. 2. SDS electrophoretic separation of CAT and SOD preparations. a) Electrophoregram of preparations and markers. Positions of protein markers (kD) are indicated on the left; bottom numbers correspond to lane numbers. Lanes: 1) bienzymic derivative of CAT and SOD treated with activated CS; 2) CAT--CS preparation; 3) native CAT; 4, 5) protein markers; 6) native SOD; 7) SOD--CS preparation. Densitograms of electrophoretic lanes/bands of the preparations: b) native CAT; c) CAT--CS derivative; d) bienzymic derivative of SOD and CAT treated with activated CS; e) SOD--CS derivative. Vertical lines correspond to the positions of the marker proteins during electrophoresis. 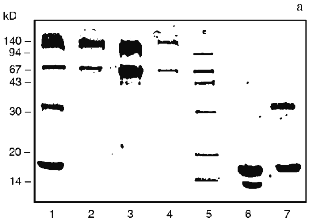
This work was supported in part by the State Scientific and Technological Program "National Priorities in Medicine and Health Care", section "Atherosclerosis" (grants No. 009 and 508) and by the Ministry of Health Care and Medical Industry of the Russian Federation.
LITERATURE CITED
1.Leo, R., Pratico, D., Iuliano, L., Pulcinelli, F.
M., Ghiselli, A., Pignatelli, P., Colavita, A. R., Fitzgerald, G. A.,
and Violi, F. (1997) Circulation, 95, 885-891.
2.Forde, R. C., and Fitzgerald, D. J. (1997)
Circulation, 95, 787-789.
3.Pratico, D., Iuliano, L., Ghiselli, A., Alessandri,
C., and Violi, F. (1991) Haemostasis, 21, 169-174.
4.Maksimenko, A. V. (1993) Usp. Sovrem. Biol.,
113, 351-365.
5.Jerondi, M. O., Hartley, C. J., and Bolli, R.
(1994) Am. J. Cardiol., 73, 2B-7B.
6.Lonn, E., Factor, S. M., Van Hoeven, K. H., Wen,
W.-H., Zhao, M., Dawood, F., and Liu, P. (1994) Can. J.
Cardiol., 10, 203-213.
7.Izawa, S., Inoue, Y., and Kimura, A. (1996)
Biochem. J., 320, 61-67.
8.Gaetani, G. F., Ferraris, A. M., Rolfo, M.,
Mangerini, R., Arena, S., and Kirkman, H. K. (1996) Blood,
87, 1595-1599.
9.Hirashi, H., Terano, A., Ota, S., Mitoh, H.,
Sugimoto, T., Razandi, M., and Ivey, K. J. (1991) Am. J.
Physiol., 261, 6921-6928.
10.Maksimenko, A. V., Bezrukavnikova, L. M.,
Grigorieva, E. L., Tischenko, E. G., Arkhipova, O. G., Yaglov, V. V.,
and Torchilin, V. P. (1992) Vopr. Med. Khim., 38, No. 3,
4-8.
11.Mao, G. D., Thomas, P. D., and Lopaschuk, G. D.
(1993) J. Biol. Chem., 268, 416-420.
12.Maksimenko, A. V., and Tischenko, E. G. (1997)
Biochemistry (Moscow), 62, 1359-1363 (Russ.).
13.Bradford, M. A. (1976) Anal. Biochem.,
72, 248-254.
14.Maksimenko, A. V., Bezrukavnikova, L. M.,
Grigorieva, E. L., Yaglov, V. V., and Torchilin, V. P. (1992) Ann.
N. Y. Acad. Sci., 672, 118-125.
15.Laemmli, U. K. (1970) Nature, 227,
680-685.