Study of Interaction of Human Replication Factor A with DNA Using New Photoreactive Analogs of dTTP
D. M. Kolpashchikov1, P. E. Pestryakov1, W. A. Wlassoff2, S. N. Khodyreva1, and O. I. Lavrik1*
1Novosibirsk Institute of Bioorganic Chemistry, Siberian Branch of the Russian Academy of Sciences, pr. Akademika Lavrent'eva 8, Novosibirsk, 630090 Russia; fax: (383-2) 333-677; E-mail: lavrik@niboch.nsc.ru2Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences, pr. Akademika Lavrent'eva 10, Novosibirsk, 630090 Russia
* To whom correspondence should be addressed.
Received August 19, 1999
Replication factor A (RPA) is a protein that binds single-stranded DNA in eukaryotic cells; it participates in replication, repair, and recombination of DNA. RPA is composed of three subunits with molecular masses 70 (p70), 32 (p32), and 14 kD (p14). The photoaffinity labeling method was used to study the interaction of RPA with the 3´-end of duplex DNA containing extended 5´-end of a single strand. We have synthesized dTTP analogs containing photoreactive 2,3,5,6-tetrafluoro-4-azidobenzoyl group attached to the 5th position of the uracil residue with linkers of variable length (9, 11, and 13 atom chains). Using these analogs and dTTP analog containing the same photoreactive residue attached to the 5th position of the uracil residue with a 4-atom linker, a number of oligonucleotide primers carrying a single photoreactive group on the 3´-end were enzymatically synthesized. Using the complex of the photoreactive primers with DNA template containing extended 19-base 5´-end, human RPA was photoaffinity modified. The primers were covalently bound to the p70 and p32 subunits of RPA and the p14 subunit was not labeled by the primers. The data are discussed considering the previously suggested model of interaction of RPA with DNA during replication.
KEY WORDS: photoaffinity modification, photoactivatable analogs of dNTP, replication protein A
RPA participates in replication, repair, and recombination of eukaryotic DNA; its main function is binding of single-stranded regions of DNA. RPA is composed of three subunits with molecular masses 70 (p70), 32 (p32), and 14 kD (p14). The p70 subunit possesses the main DNA-binding activity and is responsible for interaction with replication and repair proteins. The p32 subunit of RPA also interacts with certain proteins, for example, with uracil-DNA-glycosylase [1]. The role of the p14 subunit is poorly studied [1]. It was shown that RPA interacts with duplex DNA containing extended 5´-end of the template [2]. Uridine-5´-monophosphate residue with photoreactive 2-nitro-5-azidobenzoyl group (NAB-4-dUMP) was attached to the 3´-end of the primer by DNA polymerases; subsequent UV-irradiation resulted in covalent binding of the primer to the p32 and p70 subunits of RPA [2]. A model describing RPA interaction with duplex DNA containing extended end of the template was suggested based on the modification data. The p70 subunit was proposed to interact with single-stranded region of duplex DNA and the p32 subunit appears to be adjacent to the 3´-end of the primer, whereas the p14 subunit is far from the 3´-end or is shielded by other subunits.
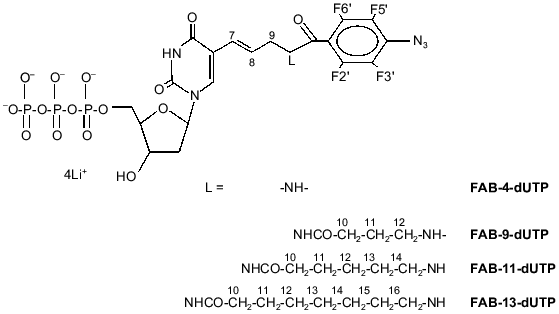
During the photolysis of the 2-nitro-5-azidobenzoyl group, initially formed singlet nitrene is rapidly transformed into the triplet state [3]; thus, triplet nitrene is the main particle reacting with the RPA subunits forming covalent bonds in these experiments. In principle, the spectrum of labeled peptides can differ when the NAB-group is substituted for another photoreactive group. Hence, it is of interest to study the modification of the RPA subunits by analogs containing other reactive groups. The perfluoroazidobenzene residue can be efficiently used for photomodification of biopolymers [4]. Photolysis of perfluoroazidophenyl groups generates singlet perfluorophenylnitrene which is the main reactive particle, and it could be covalently attached to virtually any chemical group [5]. A dTTP analog containing the 4-azido-2,3,5,6-tetrafluorobenzoyl group (FAB-4-dUTP; Fig. 1) was synthesized previously [6]. In this case, it is of interest to attach another reactive group and study the efficiency of RPA subunit labeling depending on the length of the linker connecting this group with the base.
Thus, the goal of the present work included synthesis of a number of dTTP analogs containing 4-azido-2,3,5,6-tetrafluorobenzoyl group connected by linker groups of variable length to the 5th position of the uracil residue (FAB-9-dUTP, FAB-11-dUTP, FAB-13-dUTP; Fig. 1), preparation of photoreactive primers containing the analogs and FAB-4-dUTP residue at 3´-end, and affinity modification of RPA by photoreactive primers in the presence of the template containing extended 5´-end. The main object of the work was to monitor the changes in modification efficiency of the RPA subunits depending on the length of the linker connecting the photoreactive group with 3´-end of the nucleotide primer. With the help of the dTTP analogs with long linkers, it was expected to label the p14 subunit, which was not modified by NAB-4-dUTP [2].Fig. 1. Structural formulae of FAB-n-dUTP.
MATERIALS AND METHODS
The following reagents were used: non-radioactive dNTP were from Sibenzyme (Russia); [gamma-32P]ATP was from Biosan (Russia); Rainbow molecular weight markers were from Amersham (UK); oligonucleotides including 36-base template 5´-GGTTCGATATCGTAGTTCTAGTGTATAGCCCCTACC and 16-base primer 5´-GGTAGGGGCTATACAC were synthesized at the Jacques Monod Institute (Paris, France); T4 polynucleotide kinase was from New England Biolabs (UK).
1H- and 31P-NMR spectra were recorded using a WP-200 spectrometer (Bruker, Germany) at 200 and 81 MHz, respectively, at 20°C in D2O with tetramethylsilane and 85% phosphoric acid as external reference, respectively. UV-Spectra of all photoreagents were recorded in 0.05 M Tris-HCl (pH 7.5) with a Shimadzu UV-2100 spectrophotometer (Japan) in 1 cm cells. UV-Irradiation at lambda = 315 nm was performed using a Baush and Lomb monochromator and high pressure lamp (HBO W). TLC was performed on Kieselgel 60F254 plates (Merck, Germany) in the system dioxane--water--25% aqueous ammonia (6:4:1 v/v).
5-[trans-N-(N´-(2,3,5,6-tetrafluoro-4-azidobenzoyl)-4-aminobutyryl)-3-aminopropenyl-1]-2´-deoxyuridine-5´-triphosphate (lithium salt) (FAB-9-dUTP) was synthesized as described for a similar ribose derivative [7]. The product was homogenous according to TLC (Rf 0.37). UV-Spectrum (H2O): lambdamax, nm (epsilon, M-1·cm-1) 255 (32800); NMR (2H2O) delta: 31P -19.74 (t, J = 20, Pbeta, 1P), -10.32 (d, J = 20, Palpha, 1P), -4.32 (d, J = 20, Pgamma, 1P); 1H 2.00 (m, H11, 2H), 2.39-2.48 (m, H2´, H10, 2H), 3.50 (m, H12, 2H), 3.97 (m, H9, 2H), 4.27 (m, H3´, H4´, H5´, 4H), 6.38 (m, H1´, H7, H8, 1H), 7.91 (s, H6, 1H); 19F 14.47 (d, J = 18.8, F 3.,5. 2F), 21.18 (d, J = 18.8, F 2.,6., 2F).
5-[trans-N-(N´-(2,3,5,6-tetrafluoro-4-azidobenzoyl)-4-aminohexanoyl)-3-aminopropenyl-1]-2´-deoxyuridine-5´-triphosphate (lithium salt) (FAB-11-dUTP) was synthesized as described for a similar ribose derivative [7]. The product was homogenous according to TLC (Rf 0.35). UV-Spectrum (H2O): lambdamax, nm (epsilon, M-1·cm-1) 256 (33000); NMR (2H2O) delta: 31P -19.85 (t, J = 20, Pbeta, 1P), -10.30 (d, J = 20, Palpha, 1P), -4.41 (d, J = 20, Pgamma, 1P); 1H 1.50 (m, H12, 2H), 1.73 (m, H11, H13, 4H), 2.40 (t, J = 2, H10, 2H), 2.46 (t, J = 1.5, 2H), 3.58 (t, J = 1.5, H14, 2H), 3.96 (t, J = 1.5, H9, 2H), 4.29 (m, H3´, H4´, H5´, 4H), 6.36 (m, H1´, H7, H8, 1H), 7.93 (s, H6, 1H); 19F 14.47 (d, J = 18.8, F 3.,5. 2F), 21.18 (d, J = 18.8, F 2.,6., 2F).
5-[trans-N-(N´-(2,3,5,6-tetrafluoro-4-azidobenzoyl)-4-aminooctanoyl)-3-aminopropenyl-1]-2´-deoxyuridine-5´-triphosphate (lithium salt) (FAB-13-dUTP) was synthesized as described for a similar ribose derivative [7]. The product was homogenous according to TLC (Rf 0.35; system B). UV-Spectrum (H2O): lambdamax, nm (epsilon, M-1·cm-1) 257 (33000); NMR (2H2O) delta: 31P -19.80 (s, J = 20, Pbeta, 1P), -10.26 (d, J = 20, Palpha, 1P), -4.36 (d, J = 20, Pgamma, 1P); 1H 1.41 (m, H12, H13, H14, 6H), 1.65 (m, H11, H15, 4H), 2.35 (t, J = 2, H10, 2H), 2.43 (m, H2´, 2H), 3.44 (m, H16, 2H), 3.96 (m, H9, 2H), 4.25 (m, H3´, H4´, H5´, 4H), 6.36 (m, H1´, H7, H8, 3H), 7.93 (s, H6, 1H); 19F 14.47 (d, J = 18.8, F 3.,5. 2F), 21.18 (d, J = 18.8, F 2.,6., 2F).
RESULTS AND DISCUSSION
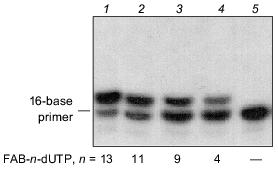
FAB-n-dUTP (n = 9, 11, 13) were synthesized according to the method described for similar ribose derivatives [7]. The structures of the compounds were confirmed by UV and NMR (1H, 31P) spectroscopy. Substrate activity of FAB-n-dUTP in the elongation reaction of 5´-32P-labeled 16-base primer catalyzed by DNA-polymerase beta was studied in the presence of 36-base oligonucleotide template. An autoradiogram of the gel after electrophoretic separation of the products of primer elongation in the presence of FAB-n-dUTP is shown in Fig. 2. In the presence of analogs (lanes 1, 2, 3, 4), only one product of primer elongation is formed and increase in the length of the linker group enhances the conversion extent of the initial nucleotide; for example, in the presence of FAB-13-dUTP, the initial 16-base primer is almost quantitatively converted to 17-base nucleotide. Thus, oligonucleotide primers are formed with one residue of FAB-n-dUMP on the 3´-end. Addition of natural dNTP to the reaction mixture (data not shown) results in generation of the products of primer elongation corresponding to 36-base oligonucleotide; hence, FAB-n-dUMP residues, when located at the 3´-end of the primer, do not inhibit primer elongation with natural substrates.
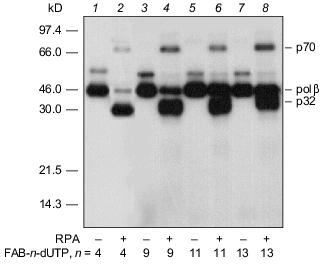
An autoradiograph of the gel after electrophoretic separation of the modification products of DNA-polymerase beta and RPA by photoreactive 5´-32P-labeled primer with 3´-terminal FAB-n-dUTP residues (n = 4, 9, 11, 13) is shown in Fig. 3. The data indicate that each analog labels DNA-polymerase beta to a similar extent. Addition of RPA inhibits labeling of DNA-polymerase beta (lanes 2, 4, 6, 8), but in case of FAB-n-dUTP (n = 11, 13) labeling is suppressed to a lesser extent (lanes 6, 8); hence, reagents with long linker groups can modify DNA-polymerase beta. This can be due to the formation of the complex DNA-polymerase beta--RPA--primer--template. In the complex, DNA-polymerase beta is located at 16-19 Å from the 3´-end of the primer. It was shown that RPA interacts with the primase subunits of the complex of DNA-polymerase alpha with primase [9]. These interactions influence the function of RPA in replication origin and during elongation. There are no data on interaction of RPA with DNA-polymerase beta, although both proteins participate in DNA repair [1]. Probably, protein--protein interaction between RPA and DNA-polymerase beta exists in the triple complex because according to electron microscopy, RPA tightly binds to single-stranded DNA region and completely occupies it transforming to an elongated shape [10]. This interaction of RPA with DNA-polymerase beta is not very tight and thus cannot be demonstrated by other methods. Chemical modification enables demonstration of weak interactions between biopolymers. Hence, interaction of RPA with DNA-polymerase beta was demonstrated in the presence of DNA. Only p32 and p70 subunits of RPA were labeled by a number of FAB-n-dUTP and NAB-4-dUTP. Thus, changes in linker length and structure of the reactive group have no effect on the pattern of modification of RPA subunits. The p14 subunit was not labeled, whereas the p32 and p70 subunits were labeled by all analogs. Thus, the data confirm the previously suggested model of RPA interaction with the 3´-end of the primer; apparently, the p70 subunit binds the single-stranded region of the template and the p32 subunit is located in close proximity to the 3´-end of the primer; the p14 subunit appears to be shielded from interaction with the 3´-end of the primer by other subunits. The leading role of p70 in binding to single-stranded extended region of duplex DNA was previously demonstrated for individual RPA subunits [11].Fig. 2. Elongation of radioactive primer by DNA-polymerase beta in the presence of FAB-n-dUTP. Reaction mixture (10 µl) contained 0.5 µM 5´-32P-labeled primer template, 1.4 µM DNA-polymerase beta, 50 mM Tris-HCl (pH 7.8), 10 mM MgCl2, 50 mM KCl, and 10 µM FAB-n-dUTP. After incubation (20 min, 25°C), the reaction mixture was treated with 10 µl 90% formamide containing 50 mM EDTA and 0.1% bromophenol blue and heated for 3 min at 80°C. Products of covalent attachment were identified by electrophoresis on 20% polyacrylamide gel under denaturing conditions with subsequent autoradiography [8].
Thus, the set of dTTP analogs containing an arylazide group attached to the base by a linker with variable length can be fruitful to study the interaction of DNA with DNA-binding proteins.Fig. 3. Photoaffinity labeling of DNA-polymerase beta and RPA by FAB-n-dUTP. All reaction mixtures contained components indicated in the legend to Fig. 2 and 0.7 µM RPA (2, 4, 6, 8). After the primer elongation (20 min, 25°C), the reaction mixture was irradiated for 15 min with 315 nm wavelength UV light and resolved by electrophoresis on 12% polyacrylamide gel with subsequent autoradiography [9].
The authors are indebted to K. Weisshart (IMB, Jena) for a gift RPA preparation. This work was supported by the Russian Foundation for Basic Research (grant Nos. 99-04-49277 and 98-04-49718), by ASGL-laboratory grant 9-7-3/SNT-99, and INTAS grant No. 96-1778.
REFERENCES
1.Wold, M. S. (1997) Ann. Rev. Biochem.,
66, 61-92.
2.Lavrik, O. I., Nasheuer, H. P., Weisshart, K.,
Wold, M. S., Prasad, R., Beard, W. A., Wilson, S. H., and Favre, A.
(1998) Nucleic Acids Res., 26, 602-607.
3.Liang, T.-Y., and Schuster, G. B. (1987) J. Am.
Chem. Soc., 109, 7803-7810.
4.Schnapp, K. A., and Platz, M. S. (1993)
Bioconjugate Chem., 4, 178-183.
5.Schuster, G. B., and Platz, M. S. (1992) Adv.
Photochem., 17, 69-143.
6.Godovikova, T. S., Kolpashchikov, D. M., Orlova, T.
N., Richter, V. A., Ivanova, T. M., Frochevsky, S. L., Nasedkina, T.
V., and Poletaev, A. I. (1999) Bioconjugate Chem., 10,
529-537.
7.Wlassoff, W. A., Kimara, M., and Ishihama, A. J.
(1999) J. Biol. Chem., 274, 5104-5113.
8.Osterman, L. A. (1981) Methods of Protein and
Nucleic Acid Research [in Russian], Nauka, Moscow.
9.Laemmli, U. K. (1970) Nature, 227,
680-685.
10.Blackwell, L. J., and Borowiec, J. A. (1996)
Mol. Cell. Biol., 16, 4798-4807.
11.Kolpashchikov, D. M., Weisshart, K., Nasheuer,
H.-P., Khodyreva, S. N., Fanning, E., Favre, A., and Lavrik, O. I.
(1999) FEBS Lett., 450, 131-134.