|
REVIEW: Is the Distribution of alpha-Tocopherol in Membranes Consistent with Its Putative Functions?P. J. QuinnDepartment of Life Sciences, King's College London, 150 Stamford Street, London SE1 9NN, U. K.; E-mail: p.quinn@kcl.ac.uk
|
Received April 30, 2003
Vitamin E acts as an antioxidant and stabilizer of membranes. Other functions of vitamin E unrelated to its effects on membranes are emerging. Vitamin E partitions into the lipid bilayer matrix of membranes. It orients perpendicularly to the plane of the membrane with the hydroxyl group pointing to the lipid-water interface. The vitamin is not randomly distributed in the plane of the membrane but tends to form clusters. These clusters appear to be composed of vitamin E and phosphatidylcholine in a stoichiometry of about one vitamin E per 10 phospholipid molecules. Vitamin E partitions into domains of phosphatidylcholine in model membranes formed from mixtures of phosphatidylcholine and phosphatidylethanolamine irrespective of whether the phosphatidylcholine is in the fluid or gel phase. The creation of domains enriched in vitamin E in membranes is not consistent with an antioxidant function and effects on membrane structure and stability indicate other roles of the vitamin.
KEY WORDS: alpha-tocopherol, phospholipid-vitamin E complexes, X-ray diffraction, membrane structure, lipid domains
Vitamin E is a fat-soluble vitamin that partitions into tissue lipids where it locates at the interface of aqueous phases and hydrophobic domains of membranes and lipoproteins. The distribution of vitamin E in the body differs from one tissue to another [1, 2]. Adipose tissue, liver, and muscle represent the major stores of vitamin E in the body, with about 90% of the vitamin being contained in the adipose tissue [3]. Of all the subcellular membrane fractions, the greatest concentrations of alpha-tocopherol are found in the Golgi membranes and lysosomes [4]. The molar ratio of alpha-tocopherol/phospholipid in these membranes is of the order of 1 : 65 phospholipid molecules, which is about an order of magnitude greater than that found in the other subcellular membranes. In all tissues, alpha-tocopherol was found to be the major form of vitamin E.
Despite its relatively low concentration compared to other membrane lipids, it is believed to play an important part in preserving the integrity of membranes. Foremost amongst its functions is its ability to protect polyunsaturated lipids of the lipid bilayer matrix of membranes against oxidation. Another important function in membranes is the formation of complexes between vitamin E and products of membrane lipid hydrolysis such as lysophospholipids and free fatty acids. The complexes thus formed tend to stabilize membranes and prevent the detergent-like actions of lipid hydrolytic products on the membrane [5].
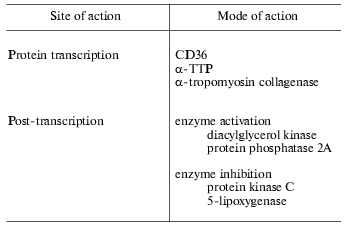
The functions of vitamin E, however, are not restricted to membrane protective roles but a variety of other actions of the vitamin have been recognized. Evidence supporting these roles has been collated by Ricciarelli et al. [6] and summarized in Table 1. The rate of transcription of certain genes such as those coding CD36, alpha-TTP, alpha-tropomyosin, and collagenase are affected by vitamin E. At the post-translational level, vitamin E has been shown to inhibit protein kinase C and 5-lipoxygenase and to activate protein phosphatase 2A and diacylglycerol kinase. Vitamin E has also been reported to inhibit cell proliferation, platelet aggregation, and monocyte adhesion. These effects are unrelated to the antioxidant activity of vitamin E, but rather are thought to result from specific interactions of vitamin E with components of the cell, such as proteins, enzymes, and membranes.
Table 1. Functions of vitamin E independent
of antioxidant and membrane stabilization (data collated from [6])
While additional actions of vitamin E cannot be entirely excluded in its overall actions in the body, in this review recent evidence relevant to the role of vitamin E in protecting membranes from free radical attack and the consequences of lipid oxidation in membranes will be examined. One of the key factors in understanding how vitamin E fulfils its functions in membranes is how it is localized in the lipid bilayer matrix and the effect its presence has on the structure and stability of membranes. Studies with model membrane systems containing vitamin E are described which address these questions.
INTERACTION OF VITAMIN E WITH PHOSPHOLIPID MODEL MEMBRANES
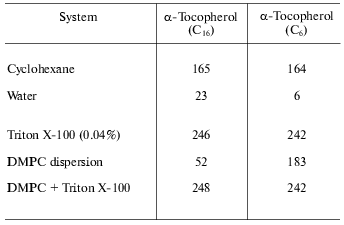
The partitioning of vitamin E into the hydrophobic domain of membranes has been inferred from the fluorescence emission properties of the molecule in environments of differing polarity. This can be seen from the fluorescence emission intensity data of the natural alpha-tocopherol (C16) compared with a shorter chain homolog (C6) in non-aqueous and aqueous model membrane systems presented in Table 2. This shows that the fluorescence intensity is relatively high in the non-polar solvent, cyclohexane, but is practically abolished by location in water. This effect is unlikely to be the result of reduction of quantum yield in the polar environment but more probably is due to self quenching of the fluorescence in insoluble aggregates of the molecule. This is consistent with the effect of detergent on fluorescence yield in which case the aggregates are solubilized resulting in a dramatic increase in fluorescence. The interesting observation is the fluorescence in dispersions of dimyristoylphosphatidylcholine. This is markedly less than in the presence of detergent suggesting that vitamin E does not distribute randomly in the phospholipid bilayer but exists in domains enriched with vitamin E. There is apparently a dependence on the length of the prenyl chain of the ability to disperse in the bilayer as the tendency of the natural homolog to associate into domains appears to be greater than that of the shorter-chain homolog.
Table 2. Fluorescence emission intensity
(arbitrary units) of 20 mM tocopherol homolog at 325 nm (data from [7])
DISTRIBUTION AND ORIENTATION OF VITAMIN E IN PHOSPHOLIPID
MEMBRANES
Three possible locations have been considered for the orientation of alpha-tocopherol in phospholipid bilayer membranes [8]. The first, based on paramagnetic resonance and other studies of alpha-tocopherol incorporated into phospholipid bilayer membranes, was a location in the region of the aqueous interface of the structure [9, 10]. The second is based on studies reported by Fragata and Bellemare [11], which indicated a location of the chromanol ring of alpha-tocopherol 1 nm from the aqueous interface. Finally, fluorescence quenching of probes located in different domains of the bilayer places the chromanol nucleus at varying depths within the bilayer structure [12].
A systematic study of the location of alpha-tocopherol and homologs with side chains of varying lengths using fluorescence quenching and fluorescence energy transfer methods has been reported by Kagan and Quinn [7]. Because the excitation and fluorescence emission wavelengths of alpha-tocopherol are appropriate for fluorescence energy transfer with anthroyloxystearate (AS) probes it was possible to obtain fairly precise distance measurements between the interacting species. The efficiency of fluorescence energy transfer between alpha-tocopherol and different n-AS probes differing in the location of the 9-anthroyloxy-group in egg phosphatidylcholine bilayers was measured and it was found that the order of efficiency followed the series 7-AS > 2-AS > 9-AS = 12-AS. This led to the conclusion that the chromanol nucleus of alpha-tocopherol is located in the bilayer in a region between the 9-anthroyloxy-group attached to carbon 7 and carbon 2 and preferentially nearest to carbon 7. Water-soluble fluorescence quenching agents were found to have low efficiency in quenching the fluorescence of alpha-tocopherol suggesting that the chromanol nucleus does not extend into the lipid-water interface.
The location of the chromanol nucleus established on the basis of fluorescence energy transfer and quenching measurements is supported by Fourier-transform infrared spectroscopy measurements [13-15]. These studies showed evidence of H-bonding of the phenoxyl hydroxy-group to either the carbonyl or phosphate oxygens of the phospholipid molecules. The possibility of hydrogen bonding to water cannot be excluded at this stage but seems unlikely. Hydrogen bonding of alpha-tocopherol to the phospholipid is consistent with bilayer permeability experiments reported by Urano et al. [16] which led them to conclude that the phenoxy-group of alpha-tocopherol is hydrogen bonded to the carbonyl group of the ester bond of unsaturated fatty acids in the phospholipids arranged in a bilayer configuration. Dynamic spectroscopic studies of alpha-tocopherol and alpha-tocotrienol indicate that the molecules rotate about their long axis perpendicular to the plane of the membrane [17].
EFFECT OF VITAMIN E ON PHOSPHOLIPID PHASE BEHAVIOR
Many studies have shown that alpha-tocopherol influences the phase behavior of phospholipids. Massey et al. [18] showed that the presence of alpha-tocopherol in aqueous dispersions of dimyristoylphosphatidylcholine decreased the temperature of the gel to liquid-crystalline phase transition and reduced the enthalpy of the transition. Similar effects were noted for fully saturated derivatives of other phospholipid classes. Other studies with unsaturated phospholipids [19], which indicated relatively minor effects of alpha-tocopherol even when present in proportions of 10 mole %, is in marked contrast with the effect on transitions from the gel phase in saturated molecular species of phospholipid where relatively large perturbations are produced.
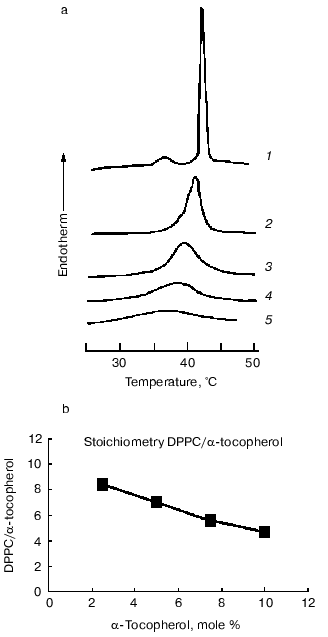
Examination of the thermal properties of mixed aqueous dispersions of dipalmitoylphosphatidylcholine with alpha-tocopherol by differential scanning calorimetry showed that increasing proportions of alpha-tocopherol cause a progressive broadening of the gel to liquid-crystalline phase transition (decrease in cooperativity of the transition) such that the onset temperature decreased but the temperature of completion of the transition was unaltered [18, 20]. A report by Villalain et al. [21] that the temperature of completion of the transition decreases with increasing proportions of alpha-tocopherol has not been confirmed in subsequent studies [22]. Proportions of alpha-tocopherol greater than about 20 mole % resulted in almost complete loss of transition enthalpy. The effect of increasing proportions of alpha-tocopherol on the endothermic transitions in heating thermograms of aqueous dispersions of dipalmitoylphosphatidylcholine is shown in Fig. 1. It can be seen that the pretransition endotherm disappears in the presence of 2.5 mole % alpha-tocopherol and the main transition progressively broadens. There is also an inverse relationship between the proportion of alpha-tocopherol in the phospholipid and the transition enthalpy. Similar findings have been reported for the dimyristoyl derivative of phosphatidylcholine [23].
It has been consistently observed in the thermal studies of mixtures of alpha-tocopherol with saturated phosphatidylcholines that the presence of alpha-tocopherol apparently eliminates the pretransition enthalpy. This does not mean that the pretransition itself is eliminated by the presence of alpha-tocopherol as it is possible that the enthalpies of the two transitions merge in the broad transition produced. Evidence for this comes from synchrotron X-ray diffraction studies of dipalmitoylphosphatidylcholine and alpha-tocopherol system, which showed that a diffraction band at about 15 nm persisted in mixtures up to at least 5 mole % alpha-tocopherol and signs of an uncoordinated ripple structure were observed at even higher proportions of alpha-tocopherol [22]. The same results were observed in systems of alpha-tocopherol with dilauroyl-, distearoyl-, and palmitoyl-oleoyl-phosphatidylcholines. This suggests that alpha-tocopherol reduces the cooperativity of the gel to liquid-crystalline phase transition but does not eliminate the pretransition, i.e., the transition from gel to ripple conformation is unaffected, at least in those molecules undergoing the transition. A reduction in overall transition enthalpy indicates that an increasing proportion of the phospholipid molecules are removed from the transition.Fig. 1. Thermal analysis of dipalmitoylphosphatidylcholine (DPPC)/alpha-tocopherol model membranes. a) Differential scanning calorimetric heating curves recorded from aqueous dispersions of dipalmitoylphosphatidylcholine and alpha-tocopherol (mole %): 1) 0; 2) 2.5; 3) 5; 4) 7.5; 5) 10. The samples contained approximately the same amount of phospholipid. No enthalpy changes were observed over this temperature range in the absence of lipid (data from [22]). b) Plot of the ratio of phospholipid molecules removed from the phase transition enthalpy calculated from the thermal data in (a) assuming that the transition enthalpy of the pure phospholipid is 35 kJ/mole per one alpha-tocopherol molecule as a function of the mole % alpha-tocopherol in the mixture. A linear regression of the form y = -0.56x + 9.6 is obtained from the plot. Numerical values are presented in Table 3 for comparison with the data obtained from X-ray measurements.
The effect of alpha-tocopherol on phase behavior of heteroacid phosphatidylcholines is complex with evidence of domain formation. Sanchez-Migallon et al. [24] examined the thermal behavior of alpha-tocopherol with 1,2-di-18-phosphatidylcholines having 18:0 fatty acids acylated in the sn-1 position and 18:0, 18:1, 18:3, or 20:4 fatty acids acylated at the sn-2 position of the glycerol. They constructed partial phase diagrams of the mixed acyl dispersions and concluded there was fluid phase immiscibility between the phospholipids and alpha-tocopherol. They concluded that lateral phase separations of domains containing different amounts of alpha-tocopherol were formed in the fluid phase. The magnitude of the effect of alpha-tocopherol on transition enthalpy was found to be dependent on the extent of unsaturation of the hydrocarbon chain located at the sn-2 position of the glycerol backbone of the phospholipid. It was suggested that alpha-tocopherol served to reduce the differences between gel and fluid states of the phospholipids, which, in turn, is related to the molecular shape of the unsaturated phosphatidylcholines.
EFFECT OF VITAMIN E ON THE STRUCTURE OF PHOSPHOLIPID MODEL
MEMBRANES
Measurements of intrinsic fluorescence of alpha-tocopherol in bilayers of dipalmitoylphosphatidylcholine suggest that alpha-tocopherol is closely associated in particular domains of the phospholipid bilayer [7, 25]. Information about the forces that may operate between phospholipids and alpha-tocopherol has come from Fourier transform infrared spectroscopy of dipalmitoylphosphatidylcholine/alpha-tocopherol mixed dispersions [21]. It was found from measurements of bandwidth and frequencies of the CH2 antisymmetric and symmetric stretch vibrations of the phospholipid acyl chains that the presence of alpha-tocopherol causes a decrease in the average number of gauche ± chain conformers at temperatures above the gel to liquid-crystalline phase transition. Interestingly, the bandwidth and frequency of maximum absorbance do not appear to change in concert with changes in temperature, which they interpreted as evidence for the coexistence of two phases. This conclusion, however, assumes that temperature has identical effects on trans-gauche isomerizations, on one hand, and lipid order and mobility, on the other.
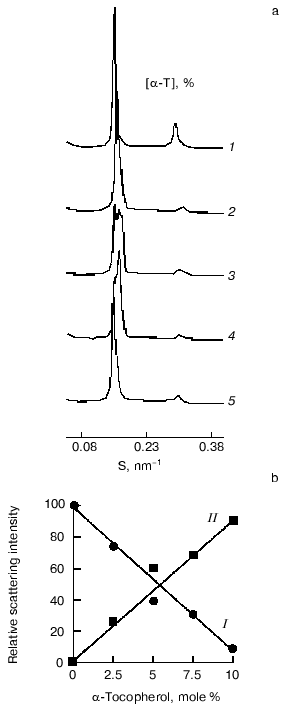
Evidence for complex formation between alpha-tocopherol and saturated phosphatidylcholines and the coexistence of these stoichiometric complexes with bilayers of pure phospholipid at temperatures below the main chain melting transition temperature have been obtained from synchrotron X-ray diffraction studies [22]. The results of such studies are presented in Fig. 2, which shows small-angle X-ray scattering intensities plotted as a function of reciprocal spacing
S = 1/d = 2sin(theta)lambda
(where d is repeat spacing, theta is diffraction angle, lambda is X-ray wavelength) recorded from codispersions of dipalmitoylphosphatidylcholine with indicated amounts of alpha-tocopherol at 47°C, a temperature above the main chain melting temperature. The pure phospholipid shows a lamellar gel phase with a single d-spacing indexed by the intense first-order lamellar reflection on the left and the smaller second-order reflection at 2/S. Multiple small-angle diffraction peaks were observed in mixed aqueous dispersions both above and below the gel to liquid-crystalline phase transition temperature of the phospholipid when alpha-tocopherol was included in the dispersion. The relative intensity of scattering of peaks centered at about 6.4 and 6.7 nm in the gel and liquid-crystal phases, respectively, decreases with increasing proportions of alpha-tocopherol. There is a corresponding increase in relative intensity of scattering of peaks centered at about 7.5 and 6.2 nm, respectively, suggesting that these spacings correspond to domains enriched in alpha-tocopherol. An increase in relative intensity of a peak centered at 8.4 nm at higher proportions of alpha-tocopherol in dipalmitoylphosphatidylcholine in the gel phase may be due to formation of a unit cell containing a higher proportion of alpha-tocopherol than that giving rise to a d-spacing of 7.5 nm. This could be due either to formation of a new phase containing an increased proportion of alpha-tocopherol or to an arrangement of domains created with lower proportions of alpha-tocopherol so as to produce a longer d-spacing. Since there are only two low-angle peaks in the fluid phase there is only one structural coordinate of the alpha-tocopherol enriched domain of dipalmitoylphosphatidylcholine. The relative scattering intensity of these two peaks is shown in Fig. 2b.
Estimates of the stoichiometry of the components of the domains enriched with respect to alpha-tocopherol were obtained from a comparison of the respective changes in phase transition enthalpy and low-angle X-ray diffraction intensities due to the presence of alpha-tocopherol in the bilayer. Calorimetric data showed that the presence of alpha-tocopherol caused a loss in cooperativity in the gel to liquid-crystal phase transition of dipalmitoylphosphatidylcholine and a decrease in transition enthalpy.Fig. 2. X-Ray diffraction analysis of dipalmitoylphosphatidylcholine-alpha-tocopherol (alpha-T) model membranes. a) Small-angle X-ray scattering intensities vs reciprocal spacing (S) recorded at 47°C from mixed aqueous dispersions of dipalmitoylphosphatidylcholine with indicated amounts of alpha-tocopherol (mole %): 1) 0; 2) 2.5; 3) 5; 4) 7.5; 5) 10 (data from [27]). b) Plots of the relative scattering intensities obtained from integrated areas of the peak in the diffraction pattern assigned to a pure phospholipid bilayer (I) and phospholipid bilayer enriched with alpha-tocopherol (II) vs the mole % of alpha-tocopherol in the mixture (data calculated from X-ray scattering intensity patterns recorded at 45°C [22]).
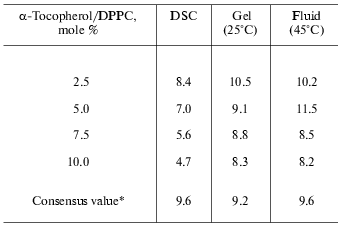
Because of the high degree of correlation between the change in excess specific heat capacity and disordering of the hydrocarbon chains, broadening of the transition with increasing alpha-tocopherol concentration indicates that the normal phase transition of the phospholipid is perturbed. It is not possible, on the basis of the present evidence, to conclude that the loss in enthalpy is due to a decrease in molar enthalpy of the phospholipid or removal of a proportion of the total phospholipid from undergoing a gel to liquid-crystal phase transition. If it is assumed, however, that domains of pure dipalmitoylphosphatidylcholine contribute normally to transition enthalpy (35 kJ/mole) then the number of molecules of dipalmitoylphosphatidylcholine that do not contribute to enthalpy in mixtures with alpha-tocopherol can be calculated from the measured transition enthalpies. When the number of dipalmitoylphosphatidylcholine molecules, that do not contribute to transition enthalpy expressed as a ratio of alpha-tocopherol molecules in the mixture, are plotted as a function of 1 mol/100 mol alpha-tocopherol in the mixture (Fig. 1), a linear regression of the form: y = -0.56x + 9.6, is obtained which indicates that increasing proportions of alpha-tocopherol result in a decrease in efficiency in removing dipalmitoylphosphatidylcholine from an endothermic transition. These DSC (differential scanning calorimetry) data are shown in Table 3. The intercept with the y-axis yields a stoichiometry of 9.6 dipalmitoylphosphatidylcholine/alpha-tocopherol. In order to account for the loss in cooperativity of the gel to liquid-crystal phase transition, a large scale phase separation of pure phospholipid and alpha-tocopherol-rich domains of phospholipid would appear to be excluded.
Table 3. Stoichiometry of
alpha-tocopherol/dipalmitoylphosphatidylcholine (DPPC)
complexes
*DSC enthalpy values from Fig. 1b; mean values
from X-ray experiments on mixtures containing 2.5, 5.0, 7.5, and 10.0
mole % alpha-tocopherol in dipalmitoylphosphatidylcholine.
Calculations of stoichiometry of alpha-tocopherol and dipalmitoylphosphatidylcholine have also been performed using the ratio of integrated intensities of the first-order lamellar repeat X-ray scattering bands recorded in mixtures in the gel phase (25°C) and the fluid phase (50°C). The results are remarkably similar to those from the thermal analysis. A summary of the enthalpy and X-ray scattering data in determination of the stoichiometry of phospholipid-alpha-tocopherol complexes is presented in Table 3. The values obtained from integrated X-ray scattering intensities in the gel phase (9.2 ± 1.1) and fluid phase (9.6 ± 1.6) suggest that the forces responsible for cohesion of the alpha-tocopherol-rich domains of phospholipid are not dependent on the phase state of the bilayer in which they are formed.
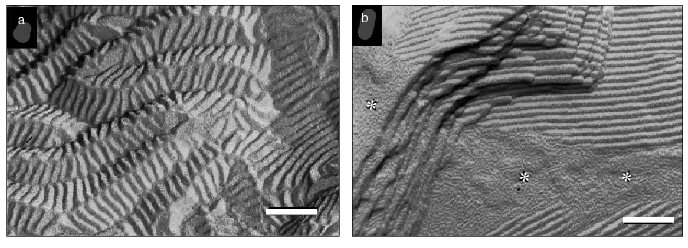
One of the manifestations of phase separation of tocopherol-enriched domains in bilayers of phosphatidylcholine is the creation of ripple or ribbon structures seen using freeze-fracture electron microscopy [26, 27]. This is illustrated in Fig. 3 which shows electron micrographs of freeze-fracture replicas prepared from codispersions of distearoylphosphatidylcholine containing 5 and 10 mole % alpha-tocopherol thermally quenched from 25°C, well below the pretransition temperature of the phospholipid, after heating from low temperature. Ripple structures can be seen which have unusually low periodicities (16 nm), which contrasts with a periodicity of 25-30 nm in the pure phospholipid in Pbeta phase observed only in the temperature range 50-54°C. It can be seen from Fig. 3a that the 16 nm symmetrically rippled areas in the dispersion containing 5 mole % alpha-tocopherol are periodically tilted forming another ripple with a large periodicity of approximately 50-150 nm running perpendicular to the small ripples. In addition to areas with straight ripples, bilayer areas were seen which show a fine, worm-like morphology (Fig. 3b, asterisk), which appear to represent disturbed ripple structure. Such features are also seen in codispersions of alpha-tocopherol with dimyristoylphosphatidylcholine [27] suggesting that beside low temperature stabilization of a symmetric ripple phase, a further effect of alpha-tocopherol is to disturb the linear order of the symmetric ripples.
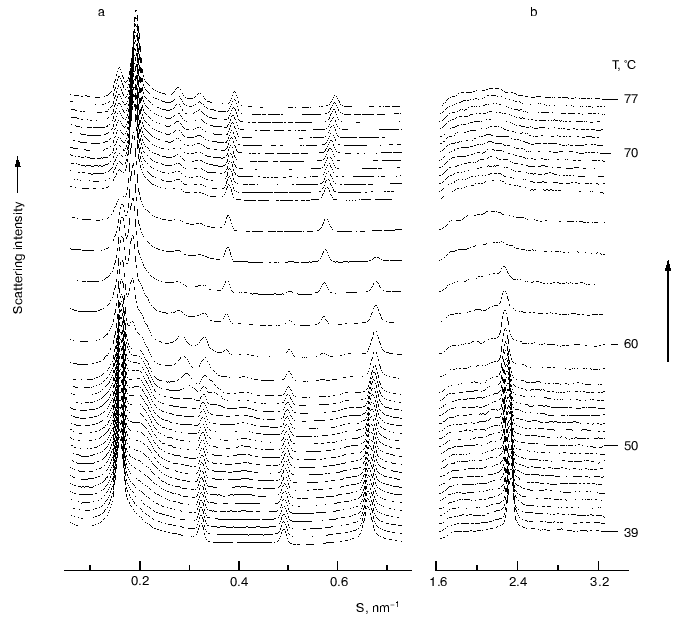
Phase separation of alpha-tocopherol rich domains has also been observed in mixtures of alpha-tocopherol and phosphatidylethanolamines [28-30]. Figure 4 shows a sequence of small- and wide-angle X-ray diffraction patterns recorded from an aqueous dispersion of dipalmitoylphosphatidylethanolamine containing 5 mole % alpha-tocopherol during a heating scan from 39-77°C. A tocopherol-enriched lamellar phase can be seen emerging as a broad diffraction band on the higher angle side of the sharp lamellar spacing originating from the pure phospholipid from which alpha-tocopherol had been excluded. An hexagonal-II phase, characterized by a set of diffraction orders in a ratio 1 : 1/v3 : 1/2, appears at a temperature of about 57°C which arises from the alpha-tocopherol rich domain and is distinct from the bilayer phase of the pure phospholipid. This suggests that alpha-tocopherol does not form a stable lamellar phase with the phosphatidylethanolamine but tends to induce hexagonal phase structure.Fig. 3. Electron micrographs of freeze-fracture replicas prepared from aqueous dispersions of distearoylphosphatidylcholine containing 5 (a) and 10 mole % (b) alpha-tocopherol thermally quenched from 25°C. Bar, 100 nm. Data from [27]. Asterisks designate bilayer areas with worm-like morphology.
Fig. 4. A sequence of small- (a) and wide-angle (b) X-ray scattering intensities vs reciprocal spacing (S) recorded from a codispersion of dipalmitoylphosphatidylethanolamine with 5 mole % alpha-tocopherol during a heating scan at 3°C/min (data from [29]).
PHASE SEPARATION OF VITAMIN E IN PHOSPHOLIPID MIXTURES
The effect of alpha-tocopherol on the phase behavior of mixed diacylphosphatidylcholines has been examined to determine whether any preferential interactions take place. Ortiz et al. [31] have studied the effect of alpha-tocopherol on the equimolar mixtures of dipalmitoylphosphatidylcholine/distearoylphosphatidylcholine (DPPC/DSPC) and dimyristoylphosphatidylcholine/distearoylphosphatidylcholine (DMPC/DSPC). The equimolar DPPC/DSPC mixture only showed a single endotherm, which was modified by the presence of alpha-tocopherol in a similar manner to the individual phosphatidylcholines. The equimolar DMPC/DSPC mixture, however, showed monotectic behavior. With increasing proportions of alpha-tocopherol the temperature range of highest temperature endotherm was broadened and shifted towards lower temperatures, while the lowest temperature endotherm was weakened and disappeared in the mixture containing 20 mole % alpha-tocopherol. This was interpreted such that alpha-tocopherol preferentially affects the lower melting component, which corresponds to DMPC. Increasing proportions of alpha-tocopherol in an equimolar mixture of 18:0/18:1- and 18:0/22:6-phosphatidylcholines also results in a two component transition enthalpy [32]. The temperature range of the lower temperature transition is progressively broadened as the alpha-tocopherol content of the mixture increases but the higher temperature component is virtually unaffected by the presence of up to 10 mole % alpha-tocopherol. This was interpreted as a phase separation of alpha-tocopherol within the mixed phospholipid dispersion with a preference for an association with the more unsaturated molecular species of phospholipid.
The inverted hexagonal phase induced by alpha-tocopherol in PEs can be used as a marker for (PE + alpha-tocopherol) domain in PC/PE mixtures [33, 34]. Synchrotron X-ray diffraction studies of mixtures of alpha-tocopherol with DOPC and DOPE showed that the effect of alpha-tocopherol on unsaturated phospholipids follows a similar pattern to that observed for their saturated counterparts. However, in the X-ray diffraction study of mixed aqueous dispersions of alpha-tocopherol with DOPE/DOPC (1 : 1) and DOPE/DMPC (1 : 1), it was found that lamellar gel and liquid-crystalline phases dominated the phase structure. This is consistent with the fact that alpha-tocopherol does not preferentially interact with PE. The changes in the SAXS patterns are similar to those of mixtures of alpha-tocopherol and phosphatidylcholines or of equimolar mixtures of PE/PC [33]. This again suggests that alpha-tocopherol preferentially interacts with the PC in the mixture or distributes randomly in domains containing both PE and PC regardless of the saturation and the length of hydrocarbon chains of the two phospholipids.
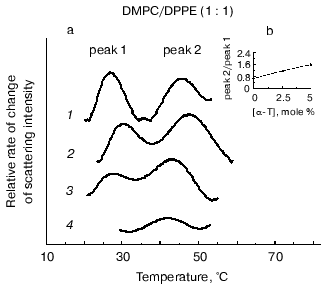
The effect of alpha-tocopherol on equimolar mixtures of saturated PE and PC has been investigated using DSC [31] and X-ray diffraction [34]. The DSC results were interpreted that alpha-tocopherol preferentially partitioned in the most fluid phase irrespective of whether this was PE or PC. However, the conclusions drawn from the X-ray data appear to be different [34]. The change in the wide-angle X-ray scattering intensity of the sharp peak at 0.43 nm has been plotted as a function of temperature in Fig. 5a for equimolar mixtures of DMPC and DPPE containing up to 20 mole % alpha-tocopherol. The curves show maxima corresponding approximately to the midpoint of the phase transition of the DMPC (peak 1) and DPPE (peak 2) components of the mixture. The position of the peaks do not correspond to identical transition temperatures in mixtures containing alpha-tocopherol because of the precise conditions that underlie the complicated processes associated with phase separation in a ternary mixture. The ratio in height, peak 2/peak 1, is found to increase with increasing alpha-tocopherol in the mixture. This is consistent with a preferential partition of alpha-tocopherol into DMPC so as to reduce the contribution of change in the intensity of the wide-angle reflection due to DMPC relative to DPPE. A similar analysis of the wide-angle X-ray scattering intensity data of mixtures of DLPE and DSPC containing different proportion of alpha-tocopherol was done and the results are presented in Fig. 5b. The inset to the figure shows the relationship between the relative heights of peaks 2 and 1 and the alpha-tocopherol (mole %) in the mixture. This indicates that as the proportion of alpha-tocopherol in the mixture increases the contribution to the change in scattering intensity of the wide-angle X-ray scattering peak from DSPC decreases relative to that from DLPE. Such data can be interpreted as a preferential partitioning of alpha-tocopherol into the high melting point phospholipid component of the mixture, which is again phosphatidylcholine, DSPC. This is consistent with the tendency of alpha-tocopherol to form complexes with phosphatidylcholines in both the fluid and gel phases and the exclusion of alpha-tocopherol from bilayer phases of phosphatidylethanolamines.
Fig. 5. Rate of change of chain packing as judged by the wide-angle scattering intensity peaks from aqueous dispersions of equimolar mixtures of DMPC/DPPE (a) and DLPE/DSPC (b) containing different mole % alpha-tocopherol (0 (1), 2.5 (2), 5 (3), 20 (4)) in the phospholipid plotted as a function of temperature. The insets show plots of the relative heights of peak 2/peak 1 as a function of the proportion of alpha-tocopherol in the mixture (data from [34]). The scattering intensities of the wide-angle peaks originating from the acyl chain packing arrangement were normalized, and the relative rate of change of scattering intensity, dI/dt, was obtained from the data recorded from heating scans of 2°C/min.
DOMAINS ENRICHED IN VITAMIN E IN MEMBRANES
There is emerging evidence that alpha-tocopherol is not randomly distributed throughout the lipid bilayer matrix of biological membranes but instead forms complexes with specific membrane constituents. The suggestion that alpha-tocopherol is preferentially located at membrane sites most susceptible to oxidation is not based on direct evidence. The formation of complexes with membrane destabilizing agents is more convincing suggesting that although present as a relatively minor membrane component it may exert a significant structural role via a localized effect. A preferential association with phosphatidylcholines as opposed to phosphatidylethanolamines may be a consequence of the fact that alpha-tocopherol forms a stable bilayer complex with phosphatidylcholine. Studies of alpha-tocopherol-phosphatidylethanolamine mixtures indicates that alpha-tocopherol is largely excluded from bilayer phases of phosphatidylethanolamine but phase separates into domains of inverted hexagonal phase of the phospholipid which would clearly have a destabilizing effect on the membrane lipid bilayer matrix. Failure to form stable complexes with phosphatidylethanolamine may be explained on the same basis as that used to explain why alpha-tocopherol readily forms complexes with free fatty acids and lysophospholipids, namely, the amphipathic balance within the complex is conducive to bilayer formation. The predominantly hydrophobic character of phosphatidylethanolamine is matched by that of alpha-tocopherol and combination of the two can only exist in non-bilayer phases.
There is clearly compelling evidence that, although present in relatively minor proportions in biological membranes, alpha-tocopherol segregates in membranes and forms complexes with specific lipid constituents. These reactions have the effect of stabilizing the lipid bilayer matrix. The wider significance of these preferential interactions, especially in regard to the putative antioxidant function of alpha-tocopherol, remains to be established.
REFERENCES
1.Hidiroglou, N., Hayward, S., Behrens, W., and
Madere, R. (1997) J. Nutr. Biochem., 8, 392-396.
2.Viana, M., Barbas, C., Castro, M., Herrera, E., and
Bonet, B. (1999) Ann. Nutr. Metab., 43, 107-112.
3.Traber, M. G., and Kayden, H. J. (1987) Am. J.
Clin. Nutr., 46, 488-495.
4.Zhang, Y., Turunen, M., and Appelkvist, E. L.
(1996) J. Nutr., 126, 2089-2097.
5.Kagan, V. E. (1988) Ann. N. Y. Acad. Sci.,
570, 120-135.
6.Ricciarelli, R., Zingg, J. M., and Azzi, A. (2002)
Biol. Chem., 383, 457-465.
7.Kagan, V. E., and Quinn, P. J. (1988) Eur. J.
Biochem., 171, 661-667.
8.Fukuzawa, K., Ikebata, W., Shibata, A., Kumadaki,
I., Sakanaka, T., and Urano, S. (1992) Chem. Phys. Lipids,
63, 69-75.
9.Perly, B., Smith, I. C. P., Hughes, L., Burton, G.
W., and Ingold, K. U. (1985) Biochim. Biophys. Acta, 819,
131-135.
10.Srivastava, S., Phadke, R. S., Govil, G., and
Rao, C. N. R. (1983) Biochim. Biophys. Acta, 734,
353-362.
11.Fragata, Y. M., and Bellemare, Y. F. (1980)
Chem. Phys. Lipids, 27, 93-99.
12.Bisby, R. H., and Ahmed, S. (1989) Free Rad.
Biol. Med., 6, 231-239.
13.Salgado, J., Villalian, J., and Gomez-Fernandez,
J. C. (1993) Eur. Biophys. J., 22, 151-155.
14.Gomez-Fernandez, J. C., Villalian, J., Aranda, F.
J., Ortiz, A., Micol, V., Coutinho, A., Berberan-Santos, M. N., and
Prieto, M. J. E. (1989) Ann. N. Y. Acad. Sci., 570,
109-120.
15.Gomez-Fernandez, J. C., Aranda, F. C., and
Villalian, J. (1991) in Progress in Membrane Biotechnology
(Gomez-Fernandez, J. C., Chapman, D., and Packer, L., eds.) Birkhauser
Verlag, Basel, pp. 98-115.
16.Urano, S., Kitahara, M., Kato, Y., Hasegawa, Y.,
and Matsuo, M. (1990) J. Nutr. Sci. Vitaminol., 36,
513-519.
17.Fukuzawa, K., Ikebata, W., Shibata, A., Kumadaki,
I., Sakanaka, T., and Urano, S. (1992) Chem. Phys. Lipids,
63, 9-75.
18.Massey, J. B., She, H. S., and Pownall, H. J.
(1982) Biochem. Biophys. Res. Commun., 106, 842-847.
19.Fukuzawa, K., Ikeno, H., Tokumura, A., and
Tsukatani, H. (1979) Chem. Phys. Lipids, 23, 13-22.
20.DeKruijff, B., van Dijck, P. W. M., Demel, R. A.,
Schuijff, A., Brants, F., and van Deenen, L. L. M. (1974) Biochim.
Biophys. Acta, 356, 1-7.
21.Villalain, J., Aranda, F. J., and
Gomez-Fernandez, J. C. (1986) Eur. J. Biochem., 158,
141-147.
22.Quinn, P. J. (1995) Eur. J. Biochem.,
233, 916-925.
23.McMurchie, E. J., and McIntosh, G. H. (1986)
J. Nutr. Sci. Vitaminol., 32, 551-558.
24.Sanchez-Migallon, M. P., Aranda, F. J., and
Gomez-Fernandez, J. C. (1996) Biochim. Biophys. Acta,
1279, 251-255.
25.Aranda, F. J., Coutinho, A., Berberan-Santos, M.
N., Prieto, M. J. E., and Gomez-Fernandez, J. C. (1989) Biochim.
Biophys. Acta, 985, 26-32.
26.Wang, X., and Quinn, P. J. (2002) Biochim.
Biophys. Acta, 1567, 6-12.
27.Wang, X., Semmler, K., Richter, W., and Quinn, P.
J. (2000) Arch. Biochem. Biophys., 377, 301-314.
28.Wang, X., Takahashi, H., Hatta, I., and Quinn, P.
J. (1999) Biochim. Biophys. Acta, 1418, 343-355.
29.Wang, X., and Quinn, P. J. (1999) Eur. J.
Biochem., 264, 1-8.
30.Wang, X., and Quinn, P. J. (2002) Chem. Phys.
Lipids, 114, 1-9.
31.Ortiz. A., Aranda, F. J., and Gomez Fernandez, J.
C. (1987) Biochim. Biophys. Acta, 898, 214-222.
32.Stillwell, W., Dallman, T., Dumaual, A. C.,
Crump, F. T., and Jenski, L. J. (1996) Biochemistry, 35,
13353-13362.
33.Wang, X., and Quinn, P. J. (2000) Biochim.
Biophys. Acta, 1509, 361-372.
34.Wang, X., and Quinn, P. J. (2000) Eur. J.
Biochem., 267, 6362-6368.