Natural Conditions Inducing Programmed Cell Death in the Yeast Saccharomyces cerevisiae
D. A. Knorre1, E. A. Smirnova1, and F. F. Severin2*
1Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, 119992 Moscow, Russia: fax: (7-095) 939-3181; E-mail: knorre@belozersky.msu.ru2BioTechnological Center, University of Technology Dresden, Tatzberg 49, D-01307 Dresden, Germany: fax: (0351) 463-40244; E-mail: severin@mpi-cbg.de
* To whom correspondence should be addressed.
Received September 28, 2004
Although yeasts have been extensively used as an experimental model to study apoptosis, it is still unclear why a unicellular organism like yeast possesses a suicide program. Here we discuss three hypothetical scenarios of “natural” yeast suicide. We argue that by correctly deducing the physiological situation(s) for yeast to undergo cell death, one can not only improve the efficiency of yeast as model system for apoptotic studies, but also obtain a certain insight into the survival strategies of communities of organisms.
KEY WORDS: yeast, programmed cell death, mitochondria, membrane potential, pheromone, reactive oxygen species (ROS), sporulation
Abbreviations: H2-DCF-DA) dichlorofluorescein diacetate; ROS) reactive oxygen species; PI) propidium iodide; DIC) differential interference contrast.
In multicellular organisms, apoptosis plays an important role during
development and in response to various pathologies. In recent years, it
has been convincingly shown that a number of unicellular organisms also
possess the ability to undergo programmed death [1-6]. In particular, the yeast
Saccharomyces cerevisiae dies in an apoptosis-like way in
response to various harsh treatments [7-13]. Still, there are two related issues concerning
programmed death in yeast that remain unclear: its physiological role
and its native inducers. It has been argued that a physiological role
of suicide in a unicellular organism is to increase the fitness and
dynamicity of the whole cell community (see [4] for
review). In other words, altruistic yeast cell death could possibly be
physiologically relevant only when yeast cells are in a community. Thus
one might speculate that, similar to bacterial suicide [3], the presence of a quorum-sensing signal in the
growth media is a necessary condition for yeast cells to commit
“physiological” suicide.
How could the yeast cell possibly sense that it is surrounded by other yeast cells? The only proven way of intercellular communication in S. cerevisiae is via sexual pheromones. Recently we have shown that the excess of these pheromones causes S. cerevisiae to die in a way resembling apoptotic death in higher cells [14]. We argued that this phenomenon might reflect programmed cell death happening in native yeast populations: elimination of a cell unable to mate when lots of mating partners are available could be beneficial for the population [5, 14].
Are there any other ways for yeast cells to sense that they are not alone but in a cell community? It has been shown that yeast in dense, chronologically aged cultures also undergo apoptosis-like death ([15-18], also see [19] for review). Interestingly, while some of the cells die in the aged cultures, the remaining actively increase their mutation rate searching for useful changes in the genome [16]. While the native inducer of cell death in this case is not defined, the gradual acidification of the media due to the accumulation of acetic acid in the aged culture (a result of glycolysis) might contribute to the quorum-sensing mechanism [16]. Consistent with that, a combination of low pH and acetate kills stationary phase yeast cells with markers of apoptosis [9]. Thus, the aged culture model seems to be physiologically relevant.
Can one possibly imagine any other natural situations when individual yeast death would be beneficial for the community? Here it is important to mention that S. cerevisiae cells are equally capable of growing either in a haploid or in a diploid form. Therefore, apart from programmed cell death, a reshuffling of the genomes by mating (haploids) and meiosis (diploids) is possible way to increase the genetic diversity and fitness of the population. As for the haploids, mating and programmed death are probably simultaneously used when the fitness of the cell community has to be improved (see above). Is the meiotic pathway also coupled to programmed death? It is known that diploids undergo meiosis (sporulate) when the conditions become unfavorable for vegetative growth. Experimental ways to induce sporulation have been extensively studied, and the requirements for efficient sporulation were found to be the following: (i) the cells have to be pre-grown on a non-fermentable carbon source to activate their mitochondria and (ii) after the pre-growth cells have to be shifted to potassium acetate media [20, 21].
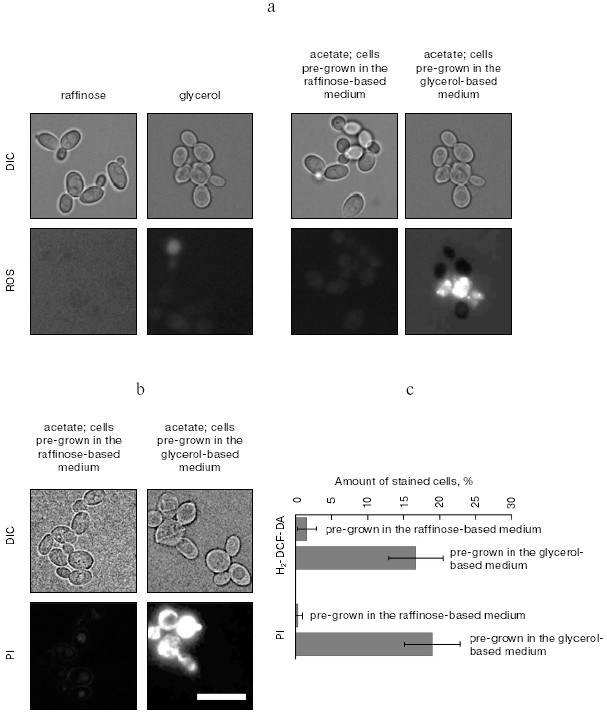
Will such a treatment induce programmed cell death in a fraction of the cells? The figure (panels (a) and (c)) shows that a culture of diploids, treated in the described way, quickly accumulates reactive oxygen species (ROS)-positive cells--a common trait of apoptosis (see [22] for review). Importantly, only cells pre-grown in the glycerol-based media accumulate ROS, while cells pre-grown in raffinose-based media and therefore having low-energized mitochondria (data not shown) are resistant to acetate-induced ROS formation (figure, panels (a) and (c)). The latter indicate that acetate does not simply intoxicate cells in a nonspecific way, but initiates the mitochondria-dependent cell death cascade. Cell death quickly follows ROS formation (figure, panels (b) and (c)). The surviving cells initiate the sporulation program and, after overnight incubation, start to form tetrads (not shown). Thus, our data suggest that the sporulation conditions can indeed initiate programmed cell death. In fact, this conclusion is not so surprising: acetate at low pH was reported to induce apoptosis-like cell death in yeast [9]. Thus, the induction of programmed cell death by acetate at pH above neutral (the pH of 2% potassium acetate is 8.0) is not entirely unexpected. Still, it is totally unclear what a possible quorum-sensing mechanism in case of sporulation-accompanied cell death could be. It is possible that, similar to the chronologically aged cultures, high concentration of acetate in the media in sporulating cultures might serve as an indicator of cell density.
To summarize, we propose that there are at least three physiologically relevant models of programmed cell death in yeast: pheromone-based, based on chronological aging, and based on sporulation media. This makes yeasts not only an instrumental system for studying the mechanisms of apoptosis, but also a promising model for studying the general laws of altruistic death of organisms. Indeed, there are two common conditions in the suggested models of “natural” suicide of yeast: an increase in the density of the cell community and a change in the environment for the worse. It is tempting to speculate that altruistic suicide in higher organisms [5, 23] could be governed by the same conditions.
REFERENCES
1.Raff, M. (1998) Nature, 396,
119-122.
2.Lam, E., Pontier, D., and del Pozo, O. (1999)
Curr. Opin. Plant. Biol., 2, 502-507.
3.Lewis, K. (2000) Microbiol. Mol. Biol. Rev.,
64, 503-514.
4.Skulachev, V. P. (2001) Exp. Gerontol.,
36, 995-1024.
5.Skulachev, V. P. (2002) FEBS Lett.,
528, 23-26.
6.Madeo, F., Engelhardt, S., Herker, E., Lehmann, N.,
Maldener, C., Proksch, A., Wissing, S., and Frohlich, K. U. (2002)
Curr. Genet., 41, 208-216.
7.Madeo, F., Frohlich, E., Ligr, M., Grey, M.,
Sigrist, S. J., Wolf, D. H., and Frohlich, K. U. (1999) J. Cell
Biol., 145, 757-767.
8.Ligr, M., Velten, I., Frohlich, E., Madeo, F.,
Ledig, M., Frohlich, K. U., Wolf, D. H., and Hilt, W. (2001) Mol.
Biol. Cell., 12, 2422-2432.
9.Ludovico, P., Sousa, M. J., Silva, M. T., Leao, C.,
and Corte-Real, M. (2001) Microbiology, 147,
2409-2415.
10.Davermann, D., Martinez, M., McKoy, J., Patel,
N., Averbeck, D., and Moore, C. W. (2002) Free Rad. Biol. Med.,
33, 1209-1220.
11.Huh, G. H., Damsz, B., Matsumoto, T. K., Reddy,
M. P., Rus, A. M., Ibeas, J. I., Narasimhan, M. L., Bressan, R. A., and
Hasegawa, P. M. (2002) Plant J., 29, 649-659.
12.Phillips, A. J., Sudbery, I., and Ramsdale, M.
(2003) Proc. Natl. Acad. Sci. USA, 100, 14327-14332.
13.Blanchard, F., Rusiniak, M. E., Sharma, K., Sun,
X., Todorov, I., Castellano, M. M., Gutierrez, C., Baumann, H., and
Burhans, W. C. (2002) Mol. Biol. Cell, 13, 1536-1549.
14.Severin, F. F., and Hyman, A. A. (2002) Curr.
Biol., 12, R233-235.
15.Herker, E., Jungwirth, H., Lehmann, K. A.,
Maldener, C., Frohlich, K. U., Wissing, S., Buttner, S., Fehr, M.,
Sigrist, S., and Madeo, F. (2004) J. Cell Biol., 164,
501-507.
16.Fabrizio, P., Pletcher, S. D., Minois, N.,
Vaupel, J. W., and Longo, V. D. (2004) FEBS Lett., 557,
136-142.
17.Fabrizio, P., Pozza, F., Pletcher, S. D.,
Gendron, C. M., and Longo, V. D. (2001) Science, 292,
288-290.
18.Fabrizio, P., Liou, L. L., Moy, V. N., Diaspro,
A., SelverstoneValentine, J., Gralla, E. B., and Longo, V. D. (2003)
Genetics, 163, 35-46.
19.Breitenbach, M., Madeo, F., Laun, P., Heeren, G.,
Jarolim, S., Frohlich, K.-U., Wissing, S., and Pichova, A. (2003) in
Model Systems in Ageing (Osiewacz, T. N. a. H. D., ed.) Vol. 3,
Springer-Verlag, Berlin-Heidelberg, pp. 61-97.
20.Codon, A. C., Gasent-Ramirez, J. M., and Benitez,
T. (1995) Appl. Environ. Microbiol., 61, 630-638.
21.Rose, M. D., Winston, F., and Hieter, P. (1990)
Laboratory Course Manuals for Methods in Yeast Genetics, Cold
Spring Harbor Laboratory Press, New York.
22.Skulachev, V. P. (1999) Mol. Aspects Med.,
20, 139-184.
23.Weismann, A. (1889) Essays upon Heredity and
Kindred Biological Problems, Claderon Press, Oxford.