REVIEW: Effect of Molecular Crowding on the Enzymes of Glycogenolysis
N. A. Chebotareva
Bach Institute of Biochemistry, Russian Academy of Sciences, Leninsky pr. 33, 119071 Moscow, Russia; fax: (495) 954-2732; E-mail: chebotareva@inbi.ras.ru
Received July 4, 2007
Cell cytoplasm contains high concentrations of macromolecules occupying a significant part of the cell volume (crowding conditions). According to modern concepts, crowding has a pronounced effect on the rate and equilibrium of biochemical reactions and stimulates the formation of more compact structures. This review considers different aspects of the crowding effect in vivo and in vitro, its role in regulation of cell volume, the effect of crowding on various interactions, such as protein-ligand and protein-protein interactions, as well as on protein denaturation, conformation transitions of macromolecules, and supramolecular structure formation. The influence of crowding arising from the presence of high concentrations of osmolytes on the interactions of the enzymes of glycogenolysis has been demonstrated. It has been established that, in accordance with predictions of crowding theory, trimethylamine N-oxide (TMAO) and betaine highly stimulate the association of phosphorylase kinase (PhK) and its interaction with glycogen. However, high concentrations of proline, betaine, and TMAO completely suppress the formation of PhK complex with phosphorylase b (Phb). The protective effect of osmolyte-induced molecular crowding on Phb denaturation by guanidine hydrochloride is shown. The influence of crowding on the interaction of Phb with allosteric inhibitor FAD has been revealed. The results show that, under crowding conditions, the equilibrium of the isomerization of Phb shifts towards a more compact dimeric state with decreased affinity for FAD.
KEY WORDS: glycogen phosphorylase b, kinase phosphorylase, molecular crowding, osmolytes, protein association, protein denaturationDOI: 10.1134/S0006297907130056
Abbreviations: PEG) polyethylene glycol; Phb) glycogen phosphorylase b; PhK) phosphorylase kinase; TMAO) trimethylamine N-oxide.
INTRODUCTION
Molecular Crowding
All biochemical processes in a cell proceed in a medium containing high concentrations of the proteins, nucleic acids, polysaccharides, and low molecular weight compounds. The total concentration of macromolecules reaches 50-400 mg/ml (for review see [1-3]), with the molecules occupying a significant part of the volume of the medium--up to 40% [1]. Such conditions in the cell are denoted as “molecular crowding”. Figure 1 demonstrates crowding conditions in the intact cell [4]. According to modern concepts, the cytoplasm is filled with large ensembles of macromolecules forming functional complexes, rather than with freely diffusing and colliding macromolecules [3]. Crowding implies the existence of nonspecific steric repulsion of molecules, which is always present regardless of any other interactions of macromolecules, for instance, electrostatic or hydrophobic. Thus, more exactly, “molecular crowding” is more accurately termed “the excluded volume effect” caused by the mutual impenetrability of cell solute molecules [5-7].
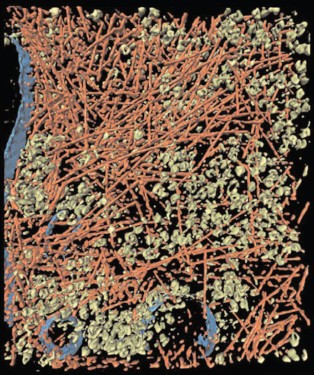
Crowding conditions in cells could arise not only from the presence of macromolecules, but also from high concentrations of osmolytes [8]. Osmolytes are low molecular weight organic compounds, such as polyols, certain amino acids, and methylamines.Fig. 1. Crowding conditions in the cytoplasm of an intact moving Dictyostelium discoideum cell. Cytoplasm is filled with macromolecular components creating crowding conditions: actin filaments (red color), ribosomes and other macromolecular complexes (green color), and membranes (blue color) (reprinted with permission from the paper [4] © 2002 AAAS).
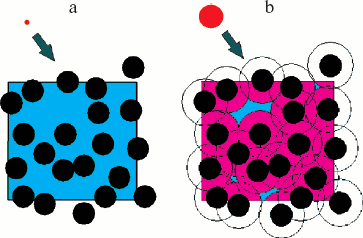
Theoretical aspects of the excluded volume effect on biochemical reactions are considered in detail in some reviews and articles [6-19]. Experimental data concerning the molecular crowding effect on the equilibrium and the rates of such biochemical processes as association, reversible denaturation, and protein folding, actin polymerization, assembly of protofibrils and fibrils, etc. studied in in vitro model systems are summarized in reviews [3, 5-9]. In all cases, the introduction of inert molecules (crowding agents) in rather high concentrations causes enhancement of interactions, increase in rates of biochemical reactions, or the shift of the equilibrium to association [5, 6, 8, 9]. The influence of crowding on the hydrodynamic and thermodynamic properties of compact globular proteins in solution could be explained by the simple model considering protein molecules as solid particles of spherical shape. The volume, which is not accessible to the center of the sphere, is excluded (Fig. 2). The larger is the volume of the molecule considered, and the higher is the concentration of the other molecules, the less is the volume accessible to the molecule in crowding conditions [6].
Excluded Volume and Thermodynamic Activity of a SubstanceFig. 2. Schematically illustrated concept of excluded volume. Squares are the volume elements containing macromolecules (black color), occupying ~30% of the total volume. The volume available (blue color) is defined as the fraction that can be occupied by the center of another molecule (red color). a) Center of the molecule of a small size could occupy almost 70% of the remaining volume (blue color). b) For center of the molecule of size comparable to the black spheres, almost all remaining volume is excluded (black and pink color) since the center of this molecule (red color) can not approach the macromolecules at the distance less than that shown by open circles (reprinted from [6] with permission).
Molecular crowding has a pronounced effect on thermodynamic activities of all soluble molecules, both large and small ones. In a solution containing macromolecules, which interact exclusively via steric repulsion, a very simple relationship between effective and real concentrations of each solute exists [6]:
gammai = ai/ci = vtot/va,i, (1)
where ai is the effective concentration or the thermodynamic activity, gammai is the activity coefficient, ci is the concentration, vtot is the total volume, va,i is the volume available to solute of type i. It is obvious that in crowding conditions gammai > 1, because va,i < vtot.
Let us consider the reversible heteroassociation A + B <--> AB. Since the intracellular medium is thermodynamically nonideal, the equilibrium constant is expressed via thermodynamic activities:
K = aAB/aAaB = gammaAB[AB]/gammaAgammaB[A][B]. (2)
In crowding conditions the apparent equilibrium constant Kapp is connected with the real constant K by the equation:
Kapp = K·Gamma, (3)
where Gamma is the nonideality factor, Gamma = (gammaAgammaB)/gammaAB. In crowding conditions, the nonideality factor could be much more than unity [11, 20]. The coefficients of the thermodynamic activity of the proteins can be evaluated according to the simple structural models where globular proteins are represented by spheres, ellipsoids, or spherocylinders [21]. By evaluating in such a way the activity coefficients of biological macromolecules and the value of the nonideality factor, it is possible to predict the effect of crowding on the equilibrium state and on the rates of biochemical reactions.
Molecular Crowding and Regulation of Cell Volume
At present, the role of crowding in supporting constant cell volume is of special interest [22]. The theory of crowding postulates that the thermodynamic activity of the protein in crowding conditions is higher than that of the same protein in equal concentration in dilute solution [11, 20]. In crowding conditions, the reaction capability of any soluble macromolecular substance is strongly dependent on the available volume [6]. The conclusion is that relatively small changes in the volume occupied by macromolecules could have a strong effect on the equilibrium and the kinetics of various intracellular reactions [6, 22, 23].
The maintenance of cell volume is therefore a fundamental physiological process and is required for cell survival [22, 24-29]. The cell volume constancy requires continued operation of regulatory mechanisms, such as ion transport through the cell membrane and accumulation or removal of organic osmolytes and metabolites. Many cell types activate the function of transporters or metabolic reactions, which are in a latent state in cells of regular volume, in response to osmotic swelling or shrinking. Cell swelling is accompanied by an increase in glycogen synthesis and by the inhibition of glycolysis [22, 30-32], by inhibition of proteolysis, and by stimulation of protein synthesis [33, 34]. Molecular crowding could be a specific mechanism that helps the cell “sense” relatively small changes in volume, and more accurately, changes in concentrations of intracellular substances [22]. According to data presented in [35-37], changes in the total concentration of cytoplasmic proteins, and, consequently, of crowding, could serve as a signal for change in cell volume. Even small changes (10%) in concentration of intracellular proteins in crowding conditions could cause a 10-fold increase in thermodynamic activity of regulatory proteins, and thereby change their capability to bind the membrane and to activate ion transporters [22].
Molecular crowding could play an important role in cell biology and physiology [3, 5-8, 22, 38-40] and also have a strong influence on the evolution of cell signaling pathways [41].
As noted above, any reactions causing an increase in available cell volume should be stimulated in crowding conditions. Reactions of such type include association of macromolecules, formation of multienzyme complexes and supramolecular structures, folding of proteins and nucleic acids, and formation of the aggregates and amyloid inclusion bodies in certain diseases (Parkinson's and Alzheimer's diseases, etc.) [3]. Thus, for instance, the interaction of glycolytic enzymes with the cytoskeletal structure is enhanced upon increase in the degree of crowding induced by protein addition. The enhancement of binding of glycolytic enzymes has also been observed, primarily the binding of phosphofructokinase, enolase, and pyruvate kinase [42]. The increase in ionic strength with the addition of salt in physiological concentrations caused only a small decrease in the stability of the enzyme binding, and the establishment of physiological concentrations of both salt and protein caused the increase in the binding of all glycolytic enzymes [43].
The goal of the present review is to discuss the effect of crowding on the glycogenolytic enzymes (glycogen phosphorylase b and phosphorylase kinase). In this review, we will consider the influence of molecular crowding, induced by high concentrations of osmolytes, on such reactions of glycogenolytic enzymes as association, isomerization, denaturation of macromolecules, formation of supramolecular structures, and interaction of macromolecules with ligands.
MOLECULAR CROWDING INDUCED BY OSMOLYTES
In response to stress (osmotic, chemical, or thermal), many living organisms accumulate high concentrations of osmolytes, which protect proteins under stress conditions [44-48] and regulate cell volume [49]. For instance, the tissues of sharks and rays contain urea in high concentration. It is known that urea causes denaturation of proteins, but this effect could be compensated through maintaining in the cell of high concentrations of counteracting osmolytes, especially such as trimethylamine N-oxide (TMAO), betaine, and sarcosine [44-48]. It was shown in vivo that TMAO counteracts the damaging action of the excess of salts and of hydrostatic pressure [50] on deep-water animals.
The effect of TMAO on protein stability and on enzymatic activity has been thoroughly studied in vitro [8, 44, 46-48, 51-56]. TMAO exists in the neutral form (zwitterionic) at pH higher than its pKa = 4.66 [51-53] and in the positively charged form at pH < pKa. In the region of pH 6.0-8.0, TMAO has a stabilizing effect on proteins [53]. The stabilizing effect of TMAO at pH > 5 and destabilizing effect of this osmolyte at pH < 5 have been demonstrated using the example of denaturation of three proteins (lysozyme, ribonuclease A, and apo-alpha-lactalbumin) [53]. The authors explain the stabilizing effect of this osmolyte mainly by “the effect of the excluded volume”, having in mind the exclusion of the osmolyte from the vicinity of the protein surface (preferential protein hydration). The destabilizing effect of the positively charged form of TMAO at pH < 5 is explained by the preferential binding of the osmolyte with the protein [53].
Some organisms accumulating proline are adapted to life under high salt concentration, and use osmolytes for compensation of the high external osmotic pressure [45]. In ocean water, the average salt concentration (mainly NaCl) is of the order of 1000 mOsm (or 1.0 Osm). In a certain group of deep-water animals (osmoconformers), the prevention of osmotic shrinking of cells is realized in such a way that the intracellular liquid has the same osmotic pressure, 1.0 Osm [49].
Cayley et al. [57] in in vivo experiments studied the growth of Escherichia coli in condition of osmotic stress over a wide range of high external osmolalities (1.02-2.17 Osm). Under conditions of osmotic stress, the amount of betaine in the cytoplasm was increased with the increase in the osmotic pressure, and the amount of K+ and biopolymers remained relatively constant. With the increase in osmolality, a significant decrease in the rate of growth of E. coli and decrease in the amount of cytoplasmic water in the cell was observed, which caused an increase in the concentration of biopolymers and all solutes, i.e. increase in crowding. Under the increase in osmolality of the external media, the concentration of betaine in the cytoplasm increases. Thus, at the external osmolality 1.02 Osm concentrations of betaine and K+ are 0.54 and 0.48 M, and at the external osmolality 2.17 Osm they are 1.67 and 0.72 M, respectively. Betaine served in this case as an osmoprotectant. On increase in osmolality the rate of E. coli growth decreases strongly, but the addition of betaine to the cultural media causes a significant increase in the period of conservation of the maximal rate of growth. The authors showed that the correlative relationship between the growth rate, the amount of cytoplasmic water, and the cytoplasmic K+ concentration is equal in the media containing betaine and in media that does not contain this osmoprotectant. According to these data, it was proposed that increase in molecular crowding, which occurs with increasing osmolality of the medium, gives the fundamental explanation both to the decrease in the growth rate and to the insensitivity of the protein-DNA interactions to the change of K+ concentration in the cytoplasm [57]. Crowding is enhanced with the increase in osmolality, which in turn buffers the binding of proteins to nucleic acids against changes in cytoplasmic K+ concentration and (by affecting biopolymer diffusion rates and/or assembly equilibria) is a determinant of growth rate of osmotically stressed cells. Changes in biopolymer concentration and crowding may also explain the increase of the activity coefficient of cytoplasmic water with increasing osmolality of growth in E. coli [57, 58].
Much experimental data has accumulated on the protective effect of osmolytes [8, 47, 48, 51-53, 59-66], but the molecular mechanisms of their action remain unclear. The same experimental data are very often explained by mechanisms that are contradictory: effect of the osmolytes is explained either as a weak interaction of osmolyte with the macromolecule (preferential binding), or as a steric repulsion, i.e. crowding effect (see review [17]).
Crowding, Preferential Hydration, and Osmotic Stress
It should be noted that there are various interpretations of the action of osmolytes on the biochemical reactions. Some researchers consider the effect of osmolytes in terms of statistical mechanics, using the theory of the excluded volume (crowding), and calculating the second virial coefficients, which characterize the interaction of the molecules in solution [12-16, 67-74]. Others use the term of preferential protein hydration [46, 47, 51, 52, 54, 59-61, 73-80], considering the layer close to the surface of the macromolecule, from which osmolyte molecules are excluded, and their place becomes occupied by the water molecules, the properties of water molecules in boundary (hydrated) layer being different in comparison to the water properties in free volume, as some authors believe [81, 82]. Winzor and Wills [12, 15] and Davis-Searles et al. [17] in their papers studied both approaches and showed that they are practically equivalent.
Parsegian et al. [83] compared three thermodynamic approaches: osmotic stress, crowding, and preferential protein hydration. Analysis of both preferential hydration and osmotic stress is based on the Gibbs-Duhem relationship [83]. In reference [83], the equivalence of these three approaches has been shown. The original papers [12, 15, 17, 77-79, 83] contain the detailed considerations, including mathematical formulas and thermodynamic parameters.
Under the modeling of molecular crowding conditions in vitro, the crowding agents are added to the solution to fill part of the volume. The main demand to these agents is that they should be neutral, and should not interact with macromolecules under test, because the crowding effect involves only steric repulsion between separate molecules. Frequently, bovine serum albumin, ovalbumin, Ficoll 70, polyethylene glycol (PEG), and osmolytes are used as crowding agents (see tables in review [8]).
We discuss below the effect of crowding induced by high concentration of osmolytes on the properties of some glycogenolytic enzymes--association and isomerization of the macromolecules, denaturation, interaction of the macromolecules with some ligands, and formation of the supramolecular structures.
EFFECT OF MOLECULAR CROWDING ON GLYCOGENOLYTIC ENZYMES
Effect of Molecular Crowding on the Interaction of Glycogen Phosphorylase b with FAD
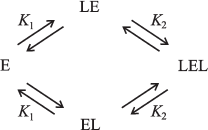
Glycogen phosphorylase b (Phb; EC 2.4.1.1) is one of the key enzymes of glycogenolysis. It catalyzes the reaction of glycogen phosphorolysis. In the resting muscle, phosphorylase exists as an inactive without AMP nonphosphorylated form b. Phb activity is regulated both by phosphorylation and by conformational changes induced by allosteric effectors. The Phb molecule is a dimer composed of two identical subunits with the molecular mass 97.4 kD [84]. Flavins (riboflavin, FMN, and FAD) are allosteric inhibitors of muscle Phb with very high affinity to the enzyme [85-89]. It is known that FAD is a dominant form among the flavins in muscle tissue. We studied the influence of molecular crowding induced by the high concentration of osmolytes--trimethylamine N-oxide (TMAO), betaine, and proline--on the interaction of muscle Phb with FAD by sedimentation velocity method using an absorbance scanning system at 20°C [90, 91]. In the absence of osmolytes, the binding of FAD with Phb is well described by one hyperbolic saturation function dependence, Y, on the concentration of free FAD, [L], and by the only dissociation constant (Kdiss) 17.8 ± 0.5 µM for two equivalent and independent sites of the dimeric enzyme molecule. In molecular crowding condition induced by osmolytes, the dependence of Y on [L] is S-shaped. The binding curve becomes progressively more sigmoidal with increasing osmolyte concentration. The binding of ligand [L] by the dimeric molecule of Phb (E) is represented in the scheme describing the interaction of FAD with the Phb molecule and considering two dissociation constants (Fig. 3).
The dissociation constant K1 characterizes the binding of the first molecule of FAD with Phb dimer. The constant K2 characterizes the binding of the second molecule of FAD with the complex of enzyme dimer with one molecule of the ligand. The effect of osmolytes on the binding of FAD with Phb is presented in the Fig. 4, which compares the saturation curves obtained in the presence of TMAO, betaine, and proline.Fig. 3. Schematic representation of FAD [L] binding by the dimeric molecule of Phb [E]. EL and LE denote 1 : 1 enzyme-ligand complexes; LEL, the completely saturated ternary complex.
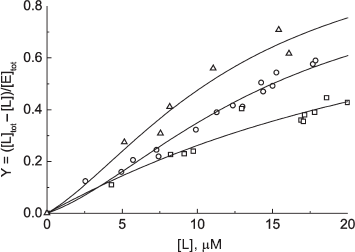
The lines drawn through the experimental points represent the best-fit approximation in terms of the relationship:Fig. 4. Fractional saturation curves for the interaction of FAD with phosphorylase b in the presence of osmolytes. The curves present the best-fit description (Eq. (4)) of results obtained in the presence 1 M concentrations of betaine (triangles), TMAO (circles), and proline (squares).
Y = ([L]/K1)(1 + [L]/K2)/(1 + 2[L]/K1 + [L]2/(K1K2)), (4)
where K1 and K2 denote the intrinsic dissociation constants describing the binding of FAD to E and EL (or LE), respectively. Magnitudes of these parameters are given in Table 1.
Table 1. Apparent dissociation constants for
the interaction of FAD with phosphorylase b [91]
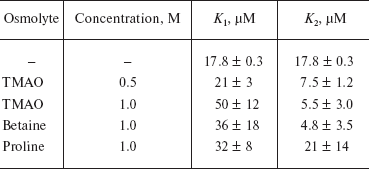
Chebotareva et al. [90] stated that in the presence of osmolytes (0.5-1.0 M TMAO, 1 M betaine, or 1 M proline) the dependence of the saturation degree of Phb dimer on the concentration of the free FAD is S-shaped. In molecular crowding conditions created by osmolytes the positive cooperative interactions of FAD-binding sites in Phb dimer appear.
The existence of FAD-induced conformational changes in the Phb molecule under molecular crowding conditions [90] is confirmed also by differential sedimentation velocity. The results show that in crowding conditions the equilibrium of the dimer isomerization is shifted to the more compact state of the enzyme with decreased affinity to FAD [90, 91].
Association of Phb in Molecular Crowding Conditions
At neutral pH, glycogen phosphorylase b (Phb) exists in the dimeric form. Conformational changes induced by binding of AMP to the allosteric activatory site favor association of Phb [84, 87]. The state of the dimer-tetramer equilibrium depends on Phb concentration, pH, ionic strength, temperature, and the presence of allosteric effectors and substrates. Glucose-1-phosphate favors association, inasmuch as glycogen and the inhibitors (ATP, ADP, flavins, etc.) prevent the formation of the tetrameric form [86-88, 92-94]. At 15°C, the interconversion dimer-tetramer is relatively slow, allowing one to separate these two species by sedimentation velocity, to determine the proportion of dimers and tetramers, and to calculate the association constant Kass in the absence and in the presence of the osmolyte. The association constant K2,4 for the dimer-tetramer equilibrium in the presence of 1 M TMAO is increased twofold [95]. The shift of the equilibrium toward the tetrameric form is also observed when PEG (37 mg/ml) with molecular mass 4000, 15,000, and 40,000 is used as a crowding agent [96].
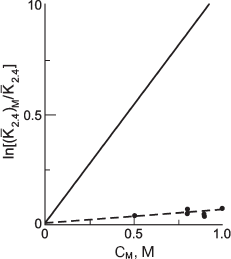
To characterize quantitatively the effect of TMAO on the association of Phb, the dependence of the logarithm of the ratio of the apparent dimerization constant (K2,4)M to the estimated one (K2,4), ln[(K2,4)M/K2,4], on the molar concentration of the osmolyte (CM) was determined and turned out to be linear (Fig. 5). The linear dependence of ln(K2,4) on CM is well predicted by the theory of excluded volume:
ln[(K2,4)M/K2,4] = (2B2,M - B4,M)CM, (5)
where second virial coefficients (B2,M for the dimer and B4,M for the tetramer) are expressed via the excluded volume together with TMAO. The molecule of TMAO is regarded as a sphere with 0.3 nm radius, RM. The values of B2,M and B4,M can be calculated by approximation of the dimer and tetramer of Phb to ellipsoids of rotation. The values of the parameters were calculated from the geometry of the molecules (the enzyme and the osmolyte) and were equal to 422 liters/mol for B2,M and 833 liters/mol for B4,M [95].
The comparison of the experimentally determined slope with the theoretical value obtained by estimation of (2B2,M - B4,M) showed that the theory of the excluded volume predicts overestimated effect. This could indicate the inadequacy of the simple dimer-tetramer model used for excluded volume calculations.Fig. 5. Dependence of the self-association behavior of phosphorylase b upon the concentration of TMAO. Effective dimerization constants are plotted in accordance with Eq. (5). The points are experimental data, the solid line is drawn in accordance with the theoretical estimation of a slope magnitude (2B2,M - B4,M).
For explanation of the data, the reversible stage of the dimer isomerization (T2 <--> R2) preceding the association (2R2 <--> R4) had to be introduced. This more appropriate model is described by the following reaction scheme:
2T2 <--> 2R2 <--> R4.
The value of the isomerization constant governing the T2 <--> R2 conversion in the presence of AMP is taken to be much smaller than unity. The consequences of including the concentration CM of inert cosolute (TMAO) are thus dual. In addition to the effect of crowding on the dimer-tetramer equilibrium, there is a compensatory effect on the isomerization equilibrium, T2 <--> R2, which is displaced towards the smaller, non-associating T state of Phb dimer [91, 95].
The expression for the dependence of the logarithm of the relationship of the measured association constant (K2,4)M to the real one (K2,4) upon TMAO concentration is described by the following relationship:
ln[(K2,4)M/K2,4] ~ [(2B2R,M - B4R,M) + 2(B2T,M - B2R,M)]CM, (6)
where the second virial coefficient for Phb dimer in T2- or R2-conformation (B2T,M and B2R,M) is expressed via the excluded volume interactions of dimer and tetramer with cosolute. The coincidence of the predicted slope with the estimated one is reached when B2T,M exceeds B2R,M by only 5 liters/mol (i.e. by 1.2% of B2T,M). The relative volume change of this magnitude (1.2%) is comparable with the corresponding value (2.8%) observed for yeast hexokinase [97].
Thus, the small increase in the degree of Phb association in the presence of TMAO, as compared with the theoretical predictions, means that the effect of molecular crowding on the dimer-tetramer equilibrium is compensated by displacing the T2 <--> R2 isomerization equilibrium for the dimer towards the more compact, non-associating T2 state.
Effect of Crowding on the Chemical Denaturation of Phb
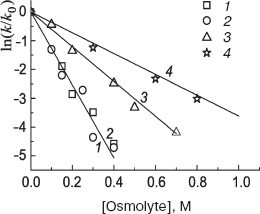
According to the predictions of the crowding theory, osmolytes in high concentrations should shift the equilibrium N <--> D (where N is the native protein and D is a denatured one) towards the native state [17]. Investigation of the influence of osmolytes on the kinetics of the chemical denaturation of Phb by guanidine hydrochloride demonstrated the protective action of TMAO, betaine, proline, and glycine [56]. Within the frameworks of the theory of the activated complex, the enzyme stability is characterized by change in the free activation energy (DeltaG0#) of the denaturation process, and the stabilizing effect is characterized by the change in the value of DeltaDeltaG0# = DeltaG0Osm# - DeltaG0# or DeltaDeltaG0# = -RTln(k/k0), where DeltaG0# and DeltaG0Osm# are the changes in the free activation energy, and k0 and k are the constants of the rate of inactivation of Phb in 0.7 M GuHCl in the absence and in the presence of the osmolyte, respectively.
The tangent of the slope on the plot of dependence of ln(k/k0) from the osmolyte concentration is the measure of protein stabilization (Fig. 6). The most pronounced stabilizing effect is exhibited by TMAO and betaine, and the effect of glycine is the least pronounced (Table 2).
Table 2. Stabilizing effect of osmolytes during phosphorylase b inactivation by 0.7 M GuHCl [56]Fig. 6. Protective effect of osmolytes--TMAO (1), betaine (2), proline (3), and glycine (4)--during phosphorylase b inactivation by 0.7 M GuHCl at 25°C.

Association of PhK under Molecular Crowding Conditions
Phosphorylase kinase (PhK; EC 2.7.1.38) plays a key role in neural and hormonal regulation of glycogenolysis in skeletal muscle. PhK catalyzes Ca2+, cAMP-dependent phosphorylation, and activation of Phb. The PhK molecule with molecular mass of 1320 kD [98-100] has a complex molecular organization and is represented by a hexadecamer consisting of four different subunits with (alphabetagammadelta)4 stoichiometry, where the gamma-subunit is the catalytic one and alpha-, beta-, and delta-subunits are the regulatory ones [101-104]. Ca2+ and Mg2+ stimulate PhK activity by inducing changes in the tertiary and quaternary structure of the molecule [102-106] and also stimulate hexadecamer association [91, 107-109]. The effect of molecular crowding on self-association of PhK from rabbit skeletal muscle has been studied by sedimentation analysis, dynamic light scattering, and turbidimetry. Sedimentation analysis demonstrated that (0.5-1.4 M) TMAO and 1 M betaine stimulate PhK association induced by Ca2+ and Mg2+. In the presence of TMAO, in addition to associates consisting of a relatively small number (n) of hexadecameric enzyme molecules (n = 2, 3, 4, ...), very large associates consisting of hundreds of PhK molecules were also registered [91, 107, 108]. The stimulating effect of TMAO on PhK association (in the presence of 0.1 mM Ca2+ and 10 mM Mg2+) was also confirmed by turbidimetric and by dynamic light scattering methods [107, 108]. The initial association rate, as measured by the turbidimetric method, increased nonlinearly with the increase of the osmolyte concentration (0.4-1.4 M) [107].
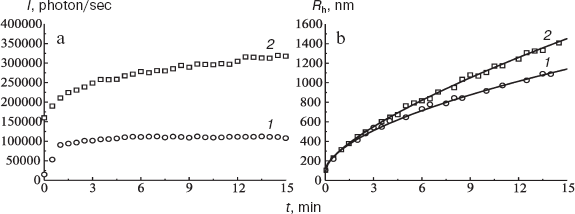
The method of dynamic light scattering allows one to estimate the hydrodynamic radius of the associates formed. As shown in Fig. 7, in the presence of 0.5 M TMAO the larger particles are formed (curve 2). Figure 7 shows only the radii of PhK associates. The radius of the PhK molecule is 13 ± 1 nm.
The enhancement of PhK association in the presence of TMAO can be explained in terms of thermodynamic non-ideality. According to the theory of absolute reaction rates, the rate constant of the second order (k) characterizing interaction between two particles (Pi + Pj → Pi+j) can be written using the following expressions:Fig. 7. Effect of TMAO on the self-association of PhK (0.3 g/liter). The dependences of the light scattering intensity (I) on time (a) and the dependences of the hydrodynamic radius (Rh) on time (b) were obtained in the absence of TMAO (1) and in the presence of 0.5 M TMAO (2).
where kB is the Boltzmann constant, T is the absolute temperature, K≠ is the equilibrium constant between the initial components (Pi and Pj, concentrations ci and cj, respectively) and the activated complex (P≠, concentration c≠). Under crowding conditions, the constant K≠ is considered as an apparent equilibrium constant:
where K0≠ is the true equilibrium constant, gammai and gammaj are the thermodynamic activity coefficients of the particles Pi and Pj, and gamma≠ is the thermodynamic activity coefficient of the activated complex. The thermodynamic activities (a) and concentrations (c) are connected by the following relationships: ai = gammaici, aj = gammajcj, and a≠ = gamma≠c≠.
The reaction rate constant k, measured in the presence of the crowding agent, is described by the following expression:
where k0 is the rate constant in the absence of the crowding agent, and Gamma is the non-ideality factor (Gamma = gammaigammaj/gamma≠). The thermodynamic activity coefficient of the solute of m-type is determined by the fraction of the total volume available to this solute:
where vtot is the total volume and vm is the volume available to the solute of m-type. It is evident that gammam > 1, because vm < vtot. Since the size of the activated complex is larger than the size of the particles Pi or Pj, the volume v≠ (the volume available to the activated complex) is less than the volume vi or vj (the volumes available to the particles Pi and Pj, respectively). Thus, gamma≠ (the coefficient of thermodynamic activity of the activated complex) is larger than gammai and gammaj (the coefficients of thermodynamic activity of the particles Pi and Pj). Since the values gammai and gammaj are larger than unity, the product gammaigammaj could be larger than gamma≠ and k > k0. This means that thermodynamic non-ideality induced by osmolytes accelerates the association of macromolecules. Such consideration allows one to explain the increase in the association rate of PhK in the presence of TMAO [108].
The effect of TMAO and betaine on PhK association is consistent with the conclusions of the excluded volume theory. The ability of PhK to form large-size associates in the medium with high concentration of osmolytes suggests that in the cell, due to the effect of molecular crowding, part of the enzyme could exist in the associated state or as supramolecular structures (as components of the protein-glycogen complex or in the bound state with the membranes of the sarcoplasmic reticulum) [110, 111].
Discussing the possible physiological significance of the formation of high molecular weight PhK associates, at present it is only possible to propose that the formation of such associates is either one of the ways of the enzyme transition from the active into the inactive form, or that the associated form might phosphorylate a substrate other than phosphorylase, for instance glycogen synthetase [110]. The authors of this paper suppose that PhK could have a dual function in glycogen biosynthesis: first, control of glycogen degradation in the protein-glycogen complex by means of Phb phosphorylation, and, second, regulation of glycogen biosynthesis on the membranes of sarcoplasmic reticulum by means of phosphorylation and, consequently, inhibition of glycogen synthetase. Further research is required to confirm or reject the second proposition.
Effect of Molecular Crowding on the Interaction of Phosphorylase Kinase with Glycogen and Phosphorylase b
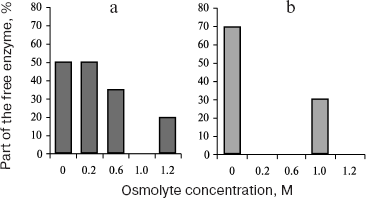
To understand the mechanism of action of glycogenolytic enzymes, it was important to study not only protein-protein interactions but also the interaction of enzymes with glycogen under crowding conditions, which imitate conditions in the cell. The influence of crowding on formation of supramolecular structures is shown by the example of PhK interaction with glycogen [112]. This is especially important, since the major part of the enzymes in muscle cells is localized in the cytoplasm in the associated state with glycogen granules [110, 113]. Using sedimentation analysis, we showed that TMAO and betaine enhanced PhK interaction with glycogen (Fig. 8). In the presence of betaine or TMAO, the fraction of free PhK registered after sedimentation of glycogen and PhK-glycogen complex decreases upon the increase in the osmolyte concentration (Fig. 8). According to turbidimetric data, betaine enhances the rate and degree of PhK interaction with glycogen [112].
The action of proline is different from that of methylamines. According to turbidimetric and sedimentation velocity data, proline prevents the formation of the protein-glycogen complex [91, 112].Fig. 8. Effect of TMAO and betaine on the binding of PhK with glycogen (40 mM Hepes, pH 6.8, 0.1 mM CaCl2, 10 mM MgCl2; 20°C). The dependence of the part of the free enzyme upon the osmolyte concentration: a) betaine at the total concentration of PhK 0.25 mg/ml, glycogen 0.9 mg/ml; b) TMAO at the total concentration of PhK 0.18 mg/ml, glycogen 0.08 mg/ml. Data obtained by sedimentation analysis method.
The influence of TMAO and betaine on PhK association and its interaction with glycogen agrees with the main postulates of the theory of molecular crowding, according to which crowding favors protein oligomerization and formation of supramolecular structures [5, 114-116]. Suppression of these processes by proline points to another mechanism of action, implying weak interaction of proline with PhK.
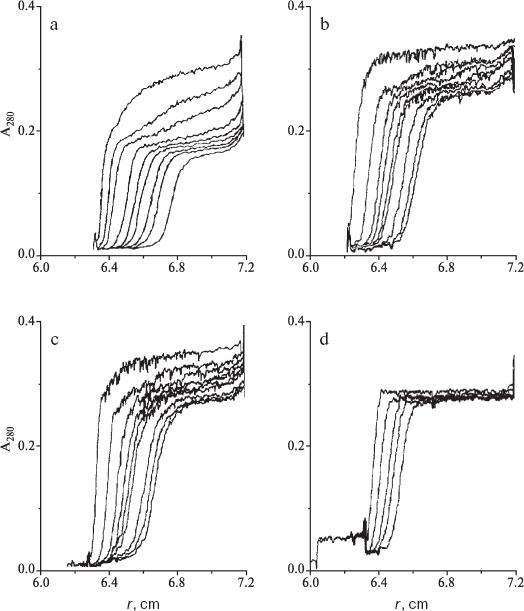
Effect of molecular crowding on interactions of phosphorylase kinase with phosphorylase b. Studies of the effect of crowding on the interaction of PhK with Phb are of special interest for several reasons. First, the physiological substrate for PhK is a protein - Phb (195 kD), and hence the enzyme-substrate interaction is a protein-protein interaction. Second, both proteins are localized on the high molecular weight matrix, glycogen particles, and interact with glycogen directly [117-122]. Each subunit of the dimeric molecule of Phb contains a specific glycogen-binding site [123]. For PhK, it was established that the alpha subunit participates in glycogen binding [124]. The presence of Phb favors PhK binding with glycogen [121, 122, 125]. Figure 9 shows the sedimentation behavior of the mixture of PhK and Phb in the absence (a) and in the presence of 0.6 M proline (b), 0.7 M betaine (c), and 0.3 M TMAO (d).
The slowly sedimenting boundary corresponds to Phb (s20,w = 8.79 S). Since the sedimentation coefficient of PhK is significantly higher than that of Phb, PhK and its complex with Phb are sedimented faster than Phb. Due to this fact, the plateau formed in the region of the meniscus after sedimentation of PhK and its complex with Phb corresponds to the concentration of the free Phb. The fraction of the free Phb increased 1.5-fold in the presence of 0.6 M proline, 0.7 M betaine, or 0.3 M TMAO. Thus, the high concentrations of proline, betaine, and TMAO completely suppress the formation of the PhK-Phb complex [91, 112].Fig. 9. Sedimentation analysis of the interaction of PhK (0.18 g/liter) with Phb (0.39 g/liter), pH 6.8, 0.1 mM Ca2+, 2.0 mM Mg2+; 20°C. Sedimentation patterns in the absence of the osmolytes (a), in the presence of 0.6 M proline (b), 0.7 M betaine (c), and 0.3 M TMAO (d). Rotor speed was 48,000 rpm. Sedimentation times were: 11, 16, 25, 32, 37, 44, 49, 58 min (a); 6, 19, 28, 33, 38, 41, 50, 57, 61 min (b); 13, 20, 29, 35, 39, 42, 51, 56, 59 min (c); 4, 10, 16, 20, 27 min (d).
Interpreting the effect of osmolytes on the PhK-Phb interaction, it should be taken into account that crowding could influence protein conformation. We showed that TMAO shifts the equilibrium of the Phb dimer isomerization reaction towards the smaller, inactive T-conformation [91, 95]. It is possible that conformational transition of Phb counteracts its interaction with PhK. However, the conditions of molecular crowding could also influence PhK conformation.
The fact that the crowding conditions prevent the formation of the complex between PhK and its substrate, Phb, may seem physiologically unreasonable at first sight. However, it was shown earlier that glycogen has a strong effect on interactions between PhK and Phb [120, 126]. We have shown that crowding conditions created by high concentration of trimethylamines favor the binding of PhK and Phb with glycogen. According to the latest data obtained by dynamic light scattering, crowding induced by 1 M TMAO strongly enhances the interaction in the tertiary complex PhK-Phb-glycogen (A. V. Meremyanin, unpublished data). Since in the living cell PhK and Phb are incorporated in the protein-glycogen complex, it is possible that specific interactions of PhK and Phb with glycogen help to overcome the negative consequences of the influence of molecular crowding on protein-protein interactions.
According to modern views, all biochemical processes in the living cell proceed under conditions of molecular crowding, which has a noticeable effect on the rate and equilibrium of various reactions and stimulates formation of more compact structures. These processes include protein-protein interactions, conformational transitions of biomacromolecules, protein folding and aggregation, and the appearance of multienzyme complexes and supramolecular structures. It is suggested that crowding plays a noticeable role in such an important physiological cell function as maintenance of constant cell volume.
For better understanding of biochemical processes in the living cell, it is important to imitate crowding conditions in in vitro experiments.
For the key enzymes of glycogenolysis, molecular crowding is a factor having a major effect on interactions such as protein-ligand and protein-protein, on conformational transitions and formation of supramolecular structures, and, as a consequence, on the mechanisms of enzyme function in vivo.
The effect of crowding on the association between PhK and Phb was discovered. It was shown that crowding stimulates PhK association in the presence of Ca2+ and Mg2+ leading to formation of supramolecular structures including hundreds of enzyme molecules. Crowding favors formation of supramolecular structures during PhK and Phb interaction with the high molecular weight matrix--glycogen. The influence of high concentrations of TMAO and betaine on the association of the enzymes of glycogenolysis, as well as on interaction of these enzymes with glycogen, is in agreement with theoretical predictions of the effects of molecular crowding.
The influence of crowding on the interaction of Phb with the allosteric effector FAD has been demonstrated. Upon interaction of Phb with FAD under crowding conditions, a conformational transition of the dimeric molecule occurs, inducing cooperative interactions of the flavin-binding sites of the enzyme.
Since the binding constants of ligands with proteins change under crowding conditions, it may be expected that crowding would influence the interaction of certain drugs with their protein targets. It is known that phosphorylase is a target for trials of certain anti-diabetic drugs [127]. Our research demonstrates that crowding effects should be taken into account during trials of new drugs.
Evaluating the possible practical significance of studies of biochemical processes under crowding conditions for medicine, it should also be taken into account that a number of diseases (such as neurodegenerative diseases) are connected with formation of non-functional protein aggregates, which is stimulated by crowding conditions.
The protective stabilizing effect of osmolytes under different kinds of stresses--thermal, chemical, or osmotic--in many cases can be explained by the effect of the excluded volume. In the present study, the protective effect of molecular crowding induced by osmolytes (TMAO, betaine, proline, and glycine) under Phb inactivation by guanidine hydrochloride has been demonstrated.
The methodical approaches to protein stabilization, based on the use of crowing effects, could be utilized to solve biotechnological problems and to develop methods for storage of medical preparations of protein nature.
The author would like to thank Prof. N. B. Livanova for critical reading of the manuscript and helpful discussions.
This work was supported by the Russian Foundation for Basic Research (grants 05-04-48691a), Russian Foundation for Basic Research and Chinese State Foundation for Natural Sciences (grant 06-04-39008), and the program “Molecular and Cell Biology” of the Presidium of the Russian Academy of Sciences.
REFERENCES
1.Fulton, A. B. (1982) Cell, 30,
345-347.
2.Zimmerman, S. B., and Trach, S. O. (1991) J.
Mol. Biol., 222, 599-620.
3.Ellis, R. J., and Minton, A. P. (2003)
Nature, 425, 27-28.
4.Medalia, O., Weber, I., Frangakis, A. S., Nicastro,
D., Gerisch, G., and Baumeister, W. (2002) Science,
298, 1209-1213.
5.Zimmerman, S. B., and Minton, A. P. (1993) Annu.
Rev. Biophys. Biomol. Struct., 22, 27-65.
6.Minton, A. P. (2001) J. Biol. Chem.,
276, 10577-10580.
7.Ellis, R. J. (2001) TRENDS Biochem.
Sci., 26, 597-604.
8.Ghebotareva, N. A., Kurganov, B. I., and Livanova,
N. B. (2004) Biochemistry (Moscow), 69, 1239-1251.
9.Hall, D., and Minton, A. P. (2003)
Biochim. Biophys. Acta, 1649, 127-139.
10.Minton, A. P. (1981) Biopolymers,
20, 2093-2120.
11.Minton, A. P. (1983) Mol. Cell.
Biochem., 55, 119-140.
12.Wills, P. R., and Winzor, D. J. (1993)
Biopolymers, 33, 1627-1629.
13.Wills, P. R, Comper, W. D., and Winzor, D. J.
(1993) Arch. Biochem. Biophys., 300, 206-212.
14.Winzor, C. L., Winzor, D. J., Paleg, L. G.,
Jones, G. P., and Naidu, B. P. (1992) Arch. Biochem.
Biophys., 296, 102-107.
15.Winzor, D. J., and Wills, P. R. (1995)
in Protein-Solvent Interactions (Gregory, R. B., ed.) Marcel
Dekker, N. Y., pp. 483-520.
16.Winzor, D. J., and Wills, P. R. (1995)
Biophys. Chem., 57, 103-110.
17.Davis-Searles, P. R., Saunders, A. J., Erie, D.
A., Winzor, D. J., and Pielak, G. J. (2001) Annu. Rev.
Biophys. Biomol. Struct., 30, 271-306.
18.Minton, A. P. (2005) J. Pharm.
Sci., 94, 1668-1675.
19.Rivas, G., Ferrone, F., and Herzfeld, J.
(2004) EMBO Rep., 5, 23-27.
20.Minton, A. P., Colclasure, G. C., and Parker, J.
C. (1992) Proc. Natl. Acad. Sci. USA, 89,
10504-10506.
21.Ellis, R. J., and Minton, A. P. (2006)
Biol. Chem., 387, 485-497.
22.Al-Habori, M. (2001) Int. J. Biochem. Cell
Biol., 33, 844-864.
23.Minton, A. P. (2000) Curr. Opin.
Struct. Biol., 10, 34-39.
24.Chamberlin, M. E., and Strange, K. (1989)
Am. J. Physiol., 257, F1-F10.
25.Sarkadi, B., and Parker, J. C. (1991)
Biochim. Biophys. Acta, 1071, 407-427.
26.Hoffmann, E. K., and Dunham, P. B. (1995)
Int. Rev. Cytol., 161, 173-262.
27.Strange, K., Emma, F., and Jackson, P. S. (1996)
Am. J. Physiol., 270, C711-C730.
28.Al-Habori, M. (1994) Int. J. Biochem.,
26, 319-334.
29.Eveloff, J. L., and Warnock, D. G. (1987)
Am. J. Physiol., 252, F1-F10.
30.Baquet, A., Hue, L., Meijer, A. J., van Woerkom,
G. M., and Plomp, P. J. A. M. (1990) J. Biol. Chem., 265,
955-959.
31.Peak, M., Al-Habori, M., and Agius, L.
(1992) Biochem. J., 282, 797-805.
32. Meijer, A. J., Baquet, A., Gustafson, L., van Woerkom, G. M.,
and Hue, I. (1992) J. Biol. Chem., 267,
5823-5828.
33.Haussinger, D., Hallbrucker, C., vom Dahl, S.,
Decker, S., Schweizer, U., Lang, F., and Gerok, W. (1991)
FEBS Lett., 283, 70-72.
34.Stoll, B., Gerok, W., Lang, F., and Haussinger,
D. (1992) Biochem. J., 287, 217-222.
35.Minton, A. P. (1997) Curr. Opin.
Biotech., 8, 65-69.
36.Garner, M. M., and Burg, M. B. (1994)
Am. J. Physiol., 266, C877-C892.
37.Parker, J. C., and Colclasure, G. C.
(1992) Mol. Cell Biochem., 114, 9-11.
38.Ellis, R. J. (1997) Curr. Biol.,
7, R531-R533.
39.Van den Berg, B., Wain, R., Dobson, C. M., and
Ellis, R. J. (2000) EMBO J., 19, 3870-3875.
40.Zimmerman, S. B. (1993) Biochim.
Biophys. Acta, 1216, 175-185.
41.Bray, D. (1998) Annu. Rev. Biophys.
Biomol. Struct., 27, 59-75.
42.Shearwin, K., Nanhua, C., and Masters, C.
(1989) Biochem. Int., 19, 723-729.
43.Shearwin, K., Nanhua, C., and Masters, C.
(1990) Biochem. Int., 21, 53-60.
44.Yancey, P. H., and Somero, G. N. (1979)
Biochem. J., 183, 317-323.
45.Yancey, P. H., Clark, M. E., Hand, S. C., Bowlus,
R. D., and Somero, G. N. (1982) Science, 217,
1214-1222.
46.Wang, A., and Bolen, D. W. (1997)
Biochemistry, 36, 9101-9108.
47.Bolen, D. W., and Baskakov, I. V. (2001) J.
Mol. Biol., 310, 955-963.
48.Burg, M. B., and Peters, E. M. (1997) Am. J.
Physiol., 273, F1048-F1053.
49.Yancey, P. H. (2005) J. Exp. Biol.,
208, 2819-2830.
50.Yancey, P. H., Fyfe-Johnson, A. L., Kelly, R. H.,
Walker, V. P., and Aunon, M. T. (2001) J. Exp. Zool.,
289, 172-176.
51.Lin, T. Y., and Timasheff, S. N. (1994)
Biochemistry, 33, 12695-12701.
52.Qu, Y., and Bolen, D. W. (2003)
Biochemistry, 36, 9101-9108.
53.Singh, R., Haque, I., and Ahmad, F. (2005)
J. Biol. Chem., 280, 11035-11042.
54.Qu, Y., Bolen, C. L., and Bolen, D. W.
(1998) Proc. Natl. Acad. Sci. USA, 95, 9268-9279.
55.Palmer, H. R., Bedford, J. J., Leader, J. P., and
Smith, R. A. J. (2000) J. Biol. Chem., 36,
27708-27711.
56.Eronina, T. B., Ghebotareva, N. A., and Kurganov,
B. I. (2005) Biochemistry (Moscow), 70, 1020-1026.
57.Cayley, S., and Record, M. T. (2003)
Biochemistry, 42, 12596-12609.
58.Cayley, S., and Record, M. T., Jr. (2004)
J. Mol. Recogn., 17, 488-496.
59.Baskakov, I. V., and Bolen, D. W. (1998)
Biophys. J., 74, 2658-2665.
60.Baskakov, I. V., and Bolen, D. W. (1998)
J. Biol. Chem., 273, 4831-4834.
61.Liu, Y., and Bolen, D. W. (1995)
Biochemistry, 34, 12884-12891.
62.Mashino, T., and Fridovich, I. (1987) Arch.
Biochem. Biophys., 258, 356-360.
63.Meng, F.-G., Park, Y.-D., and Zhou, H.-M. (2001)
Int. J. Biochem. Cell Biol., 33, 701-709.
64.Satoro, M. M., Liu, Y., Khan, S. M. A., Hou,
L.-X., and Bolen, D. W. (1992) Biochemistry, 31,
5278-5283.
65.Yancey, P. H., and Somero, G. N. (1980)
J. Exp. Zool., 212, 205-213.
66.Zou, Q., Bennion, B. J., Daggett, V., and Murphy,
K. P. (2002) J. Am. Chem. Soc., 124,
1192-1202.
67.Poon, J., Bailey, M., Winzor, D. J., Davidson, B.
E., and Sawyer, W. H. (1997) Biophys. J., 73,
3257-3264.
68.Patel, C. N., Noble, S. M., Weatherly, G. T.,
Tripathy, A., Winzor, D. J., and Pielak, G. J. (2002) Prot.
Sci., 11, 997-1003.
69.Wills, P. R., Jacobsen, M. P., and Winzor, D.
J. (1996) Biopolymers, 38, 119-130.
70.Lonhienne, T. G. A., and Winzor, D. J.
(2001) Biochemistry, 40, 9618-9622.
71.Lonhienne, T. G. A., and Winzor, D. J. (2002)
Biochemistry, 41, 6897-6901.
72.Jacobsen, M. P., Wills, P. R., and Winzor, D. J.
(1996) Biochemistry, 35, 13173-13179.
73.Hall, D. R., Jacobsen, M. P., and Winzor, D.
J. (1995) Biophys. Chem., 57, 47-54.
74.Wills, P. R., Hall, D. R., and Winzor, D. J.
(2000) Biophys. Chem., 84, 217-225.
75.Timasheff, S. N. (1993) Annu. Rev. Biophys.
Biochem. Struct., 22, 67-97.
76.Baskakov, I. V., and Bolen, D. W. (1998)
Biophys. J., 74, 2666-2673.
77.Timasheff, S. N. (1998) Proc. Natl.
Acad. Sci. USA, 95, 7363-7367.
78.Timasheff, S. N. (2002)
Biochemistry, 41, 13473-13482.
79.Timasheff, S. N. (2002) Proc. Natl.
Acad. Sci. USA, 99, 9721-9726.
80.Qu, Y., and Bolen, D. W. (2002)
Biophys. Chem., 101, 155-165.
81.Despa, F., Fernandez, A., and Berry, R. S.
(2004) Phys. Rev. Lett., 93, 228104.
82.Despa, F., Orgill, D. P., and Lee, R. C.
(2005) Ann. N. Y. Acad. Sci., 1066, 54-66.
83.Parsegian, V. A., Rand, R. P., and Rau, D. C.
(2000) Proc. Natl. Acad. Sci. USA, 97, 3987-3992.
84.Barford, D., and Johnson, L. N. (1989)
Nature, 340, 606-616.
85.Chebotareva, N. A., Kurganov, B. I., Pekel, N.
D., and Beresovskii, V. M. (1986) Biochem. Int., 13,
189-197.
86.Chebotareva, N. A., Kurganov, B. I., Lubarev, A.
E., Davydov, D. R., and Pekel, N. D. (1991) Biochimie,
73, 1339-1343.
87.Chebotareva, N. A., Klinov, S. V., and Kurganov,
B. I. (2001) Biotechnol. Genet. Eng. Rev., 18,
265-297.
88.Kurganov, B. I., Klinov, S. V., and Chebotareva,
N. A. (1994) Uspekhi Biol. Khim., 34, 83-110.
89.Kurganov, B. I., Schors, E. I., Livanova, N. B.,
Chebotareva, N. A., Eronina, T. B., Andreeva, I. E., Makeeva, V. F.,
and Pekel, N. D. (1993) Biochimie, 75,
481-485.
90.Chebotareva, N. A., Kurganov, B. I., Harding, S.
E., and Winzor, D. J. (2005) Biophys. Chem., 113,
61-66.
91.Chebotareva, N. A. (2006) Interaction of
Glycogenolytic Enzymes under Molecular Crowding Conditions: Doctoral
dissertation [in Russian], Bach Institute of Biochemistry, Moscow,
p. 50.
92.Sprang, S. R., Acharya, K. R., Goldsmith, E. J.,
Stuart, D. L., Varvill, K., Fletterick, R. J., Madsen, N. B., and
Johnson, L. N. (1988) Nature, 336, 219-221.
93.Sprang, S. R., Withers, S. G., Goldsmith, E. J.,
Fletterick, R. J., and Madsen, N. B. (1991) Science,
254, 1367-1371.
94.Livanova, N. B., and Kornilaev, B. A. (1996)
Biochemistry (Moscow), 61, 1432-1442.
95.Chebotareva, N. A., Harding, S. E., and Winzor,
D. J. (2001) Eur. J. Biochem., 268, 506-513.
96.Kurganov, B. I., Topchieva, I. N., Lisovskaya, N.
P., Chebotareva, N. A., and Natarius, O. Ya. (1979)
Biokhimiya, 44, 629-633.
97.Jacobsen, M. P., and Winzor, D. J. (1997)
Progr. Colloid Polym. Sci., 107, 82-87.
98.Krebs, E. G., Graves, D. J., and Fisher, E. H.
(1959) J. Biol. Chem., 23, 2867-2873.
99.Brusharia, R. J., and Walsh, D. A. (1999)
Front. Biosci., 4, 618-641.
100.Livanova, N. B. (1993) Biochemistry
(Moscow), 58, 1234-1239.
101.Cohen, P. (1973) Eur. J.
Biochem., 34, 1-14.
102.Nadeaw, O. W., Traxler, K. W., Fee, L. R.,
Baldwin, B. A., and Carlson, G. M. (1999) Biochemistry,
38, 2551-2559.
103.Wilkinson, D. A., Fitzgerald, T. J., Marion, T.
N., and Carlson, G. M. (1999) J. Protein Chem., 18,
157-164.
104.Priddy, T. S., Middaugh, C. R., and Carlson, G.
M. (2007) Protein Sci., 16, 517-527.
105.Priddy, T. S., MacDonald, B. A., Heller, W. T.,
Nadeau, O. W., Trewhella, J., and Carlson, G. M. (2005) Protein
Sci., 14, 1039-1048.
106.Priddy, T. S., Price, E. S., Johnson, C. K.,
and Carlson, G. M. (2007) Protein Sci., 16,
1017-1023.
107.Chebotareva, N. A., Andreeva, I. E., Makeeva,
V. F., Kurganov, B. I., Livanova, N. B., and Harding, S. E. (2002)
Progr. Colloid Polym. Sci., 119, 70-76.
108.Chebotareva, N. A., Meremyanin, A. V., Makeeva,
V. F., and Kurganov, B. I. (2006) Progr. Colloid Polym.
Sci., 131, 83-92.
109.Meremyanin, A. V., Chebotareva, N. A., Makeeva,
V. F., and Kurganov, B. I. (2007) Dokl. Ros. Akad. Nauk,
415, 1-3.
110.Polishchuk, S. V., Brandt, N. R., Meyer, H. E.,
Varsanyi, M., and Heilmeyer, L. M., Jr. (1995) FEBS
Lett., 362, 271-275.
111.Singh, P., Salih, M., Leddy J. J., and Tuana,
B. S. (2004) J. Biol. Chem., 279, 35176-35182.
112.Chebotareva, N. A., Andreeva, I. E., Makeeva,
V. F., Livanova, N. B., and Kurganov, B. I. (2004) J. Mol.
Recogn., 17, 426-432.
113.Meyer, H. E., Heilmeyer, L. M. G., Jr., and
Haschke, R. H. (1970) J. Biol. Chem., 245, 6642-6648.
114.Shearwin, K. E., and Winzor, D. J. (1988)
Biophys. Chem., 31, 287-294.
115.Cann, J. R., Coombs, R. O., Howlett, G. R.,
Jacobsen, M. P., and Winzor, D. J. (1994) Biochemistry,
33, 10185-10190.
116.Shtilerman, M. D., Ding, T. T., and Lansbury,
P. T., Jr. (2002) Biochemistry, 41, 3855-3860.
117.Chebotareva, N. A., Lisovskaya, N. P., and
Kurganov, B. I. (1979) Mol. Biol. (Moscow), 13,
228-236.
118.Klinov, S. V., Chebotareva, N. A., Lisovskaya,
N. P., Davidov, D. R., and Kurganov, B. I. (1982) Biochim.
Biophys. Acta, 709, 91-98.
119.Steiner, R. F., and Marshall, L. (1982)
Biochim. Biophys. Acta, 707, 38-45.
120.Zemskova, M. A., Shur, S. A., Skolysheva, L.
K., and Vulfson, P. L. (1989) Biokhimiya, 54,
662-668.
121.Andreeva, I. E., Makeeva, V. F., Kurganov, B.
I., Chebotareva, N. A., and Livanova, N. B. (1999) FEBS Lett.,
445, 173-176.
122.Andreeva, I. E., Makeeva, V. F., Kurganov, B.
I., Chebotareva, N. A., and Livanova, N. B. (1999) Biochemistry
(Moscow), 64, 159-168.
123.Oikonomakos, N. G., Acharya, K. R., and
Johnson, L. N. (1992) Post-Translational Modifications of
Proteins (Harding, J. J., and Crabbe, M. J. C., eds.) CRC Press,
Boca Raton, FL, pp. 81-151.
124.Chan, K. F., and Graves, D. J. (1982) J.
Mol. Biol., 257, 5939-5947.
125.Shmelev, V. K., and Serebrenikova, T. P. (1997)
Biochem. Mol. Biol. Int., 43, 867-872.
126.Morange, M., and Buc, H. (1979)
Biochimie, 61, 633-643.
127.Oikonomakos, N. G. (2002) Curr.
Protein Pept. Sci., 3, 561-586.