Mechanism of Photoisomerization of the Rhodopsin Chromophore
N. S. Vassilieva-Vashakmadze*, R. A. Gakhokidze, and A. R. Gakhokidze
Javakhishvili Tbilisi State University, pr. Chavchavadze 1, Tbilisi, Georgia; E-mail: nonavas@rambler.ru; tamazvashakmadze@yahoo.com; rgakhokidze@mail.ru* To whom correspondence should be addressed.
Received November 6, 2006; Revision received October 25, 2007
This work is devoted to the problem connected with rhodopsin activation. Electrostatic forces involved in photoisomerization of retinal are considered. It is suggested that the repulsion force and rotating moment between electric dipole moments of methyl groups on the C5 and C13 atoms of retinal can promote isomerization upon light absorption because the pi-pi* transition is accompanied by a decrease in the potential barrier for torsional rotations around the C11-C12 bond.
KEY WORDS: visual receptor, diamagnetism, dipole moment, rotating moment, isomerizationDOI: 10.1134/S0006297908060151
Vision involves a series of processes starting from electromagnetic wave perception in light-sensitive eye receptors (rhodopsin of rods and iodopsin of cones). The literature contains a large body of experimental data concerning the structure and function of visual receptors (particularly, rhodopsin). The mechanism of rhodopsin isomerization was reviewed elsewhere [1-5]. Steric repulsion between hydrogen atom of the C10 atom and the methyl group of C13 was shown to modify the quantum yield of the isomerization. Side methyl groups of C5 and C13 (polarization of the bond between side methyl group and carbon atom of the polyene chain) should also be taken into account, because they have an effect on cis-trans isomerization of retinal.
To provide more detailed description of the visual process, the spatial configuration of the electrostatic field in the cavity (pocket) in the protein moiety of rhodopsin (opsin) should be taken into account. The chromophore (11-cis-retinal) is located within this cavity. The chromophore interacts with the protein moiety, and photoinduced chromophore isomerization modifies the protein part of the receptor [5].
The goal of this work was to consider the mechanism of the fastest primary reaction induced by a quantum of light (isomerization or rearrangement of the nuclear subsystem of retinal) coupled with electronic rearrangement of 11-cis-retinal. Further steps of the reaction cascade in rhodopsin (signal transmission from retinal to opsin) are beyond the scope of this work. A semiclassical approach to this problem was sufficient to obtain qualitative results. Our task was to analyze the properties of the electromagnetic wave interacting with visual receptor chromophore and intramolecular processes in the receptor itself. The intramolecular processes in the receptor modify the state of the ion channel associated with the receptor.
An electromagnetic wave of the optical range is absorbed by the receptor chromophore in the first stage of the visual process. The chromophores with electric dipole moment parallel to the electric vector of the incident light absorb the light with linear polarization [6].
Retinal (the chromophore of rod rhodopsin) in the dark is oriented virtually across the ion channel formed by the subunits of the opsin (protein moiety of the receptor) (Fig. 1). It is well known that the pi-electron system of the rhodopsin chromophore group (11-cis-retinal) is delocalized [6-8], and light induces pi→pi* transition of the electronic system with subsequent cis-trans isomerization of the retinal. The energy of the transition is about 2 eV.
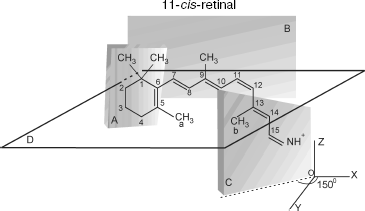
The transition induces electronic density redistribution on retinal atoms and bonds. The electronic density at the C12-C13 bond and atom C12 decreases slightly, whereas the electronic density at the retinal ends increases. An increase in the electronic charge at the nitrogen atom weakens the bond between the retinal tail fragment and a lysine residue of opsin [8].Fig. 1. Spatial configuration of 11-cis-retinal according to [12]. Plane A, in which the indole ring is situated, forms an angle -65° with respect to plane B, in which the polyene chain is situated. Plane C, in which the tail fragment of 11-cis-retinal is situated, forms an angle +150° with respect to plane B.
Light-induced cis-trans isomerization of retinal is characterized by high stereospecificity, fast reaction rate (reaction time, ~200 fsec), and high quantum yield (~0.67) [3]. Cis-trans isomerization is believed to be caused by weakening of the C12-C13 bond. However, rotation is thought to be implemented not only by weakening of force constant of the bond. It is obvious that it requires also rotating moment causing the turn. Let us consider this opportunity in more detail.
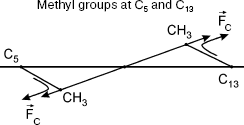
11-cis-Retinal contains five methyl groups. The distance between methyl groups (a) and (b), r12, is approximately 4.5 Å. Methyl groups (a) and (b) are connected to C5 of the cyclohexane ring and C13 of the retinal tail fragment, respectively (Fig. 1).
11-cis-Retinal configuration is non-planar, which was reported for bovine rhodopsin [8]. Therefore, methyl groups of C13 and C5 atoms are above and below plane B, respectively. The polyene chain segment is localized in plane B (Fig. 2).
Substitution of the rhodopsin chromophore by a retinal analog with 5,6-dehydroretinal derivative was shown to inhibit activity as measured using circular dichroism [8]. This indicates reduced isomerization activity of the rhodopsin derivative with methyl group of C5 substituted by hydrogen. This also indicates the role of the CH3-C5 bond in light-induced isomerization of 11-cis-retinal.Fig. 2. Methyl groups (a) and (b) are below and above plane B, respectively.
Side methyl groups in unsaturated molecules produce electric dipole moment with main chain atoms. Side methyl groups are characterized by electron density deficiency, and dipole moment is directed from the C atom of the main chain toward the side methyl group [9]. According to the literature [9-11], the electric dipole moment associated with the side methyl group and the carbon atom of the polyene chain is:
where D is dipole moment, D is Debye (1 D = 10-18 CGS) [9]. The initial angle between dipole moments (a) and (b) is about 135° [12]. The energy U, force F, and rotating moment N characterize the interaction between electric dipole moments (a) and (b) [13, 14]:
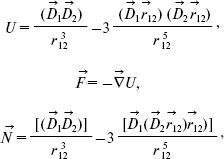 (2)
(2)where D1 and D2 are dipole moments of groups (a) and (b), respectively, r12 is the distance between the dipole moments.
Substituting Eq. (1) into Eqs. (2) and assuming that r12 = 4.5 Å, numerical values of these variables are obtained:
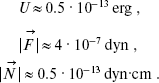 (3)
(3)
The force constant of torsional oscillations is described by an equation derived in [14]:
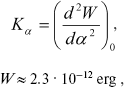 (4)
(4)where W is potential barrier, alpha is angle of torsional oscillations.
According to [14], the force constant is Kalpha ~ 3·104 CGS. Therefore, W ~ 3.9·10-12 erg. Thus, the potential barrier of torsional oscillations of 11-cis-retinal with respect to bond C12-C13 inhibits intramolecular rotation in retinal (because U (Eq. (3)) < W (Eq. (4)).
The pi→pi* transition induced by absorption of a quantum is accompanied by electron density redistribution in the 11-cis-retinal, thereby changing the force constant (Kalpha → K*alpha) of torsional oscillations around the C11-C12 bond. It should be noted that K*alpha < Kalpha.
The decrease in the force constant correlates with the decrease in the potential barrier of torsional oscillations with respect to bond C11-C12. According to the literature [15, 16], exposure to light induces a decrease in the potential barrier W* of torsional oscillations around the C11-C12 bond. This decrease converts the double bond into an ordinary single bond during the pi→pi* transition. The potential barrier of torsional oscillations is ~5-6 kcal/mol. Therefore, W* < 0.25?10-12 erg. According to [5], exposure to light reduces the potential barrier of torsional oscillations around the C11-C12 bond virtually to zero.
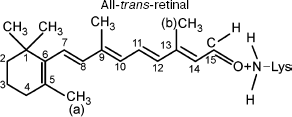
It should be taken into account that the pi→pi* transition significantly modifies the properties of the pi-electron system. The initial properties of the side methyl groups (a) and (b) remain invariable because the pi→pi* transition modifies only the pi-electron system rather than side CH3 groups. The side CH3 groups do not contribute to formation of the pi-electron system [9, 14]. The effective charges and electric dipole moments of the side groups remain invariable. Therefore, in this case the energy of dipole-dipole repulsion U (Eq. (3)) is sufficient for overcoming the potential barrier of torsional oscillations with respect to bond W (C11-C12). Thus, the force of dipole-dipole repulsion F (Eq. (3)) and torsional moment N (Eq. (3)) are able to induce rotation around the C11-C12 bond. The final configuration in this case may correspond to a new steady-state conformation (all-trans-retinal) (Fig. 3).
This work was supported by the National Scientific Foundation of Georgia.Fig. 3. Chromophore group of rhodopsin (all-trans-retinal) after exposure to light.
REFERENCES
1.Cviklinski, A. R., and Tajbaksh, E. M. (2002)
Eur. Phys. J., E9, 427-434.
2.Potapov, V. M. (1988) Stereochemistry [in
Russian], Nauka, Moscow.
3.Kalamkarov, G. R., and Ostrovsky, M. A. (2002)
Molecular Mechanisms of Visual Reception [in Russian], Nauka,
Moscow, p. 279.
4.Peteanu, L. A., Schoenlein, R. W., Qing Wang,
Mathies, R. A., and Shank, Ch. V. (1993) Proc. Natl. Acad.
Sci. USA, 90, 11762-11766.
5.De Grip, W., de Lange, F., Bovee, P., Verdegem, P.
J. E., and Lugtenburg, J. (1997) Pure & Appl. Chem.,
89, 2091-2098.
6.Zimmerman, A. L. (1998) Cell Physiology Source
Book (Sperelakis, N., ed.) 2nd Edn., University of Cincinnati, pp.
718-725.
7.Zuker, C. S. (1996) Proc. Natl. Acad. Sci.
USA, 93, 571-576.
8.Kosower, E. M. (1998) Molecular Mechanisms for
Sensory Signals, Princeton University Press, Princeton, NJ,
USA.
9.Neiland, O. Ya. (1990) Organic Chemistry [in
Russian], Vysshaya Shkola, Moscow.
10.Keshtova, S. V. (2004) Analysis of Empirical
and Non-empirical Methods of Determination of Partial Charge of Atoms
and Molecules [in Russian], Moscow State University Publishers
GROMOS 96.
11.Kalugin, O. N., Pazyura, Yu. I., and Kolesnik,
Ya. V. (2005) Khimiya, 13 (36), No. 669, All-Russian
Chemical Research Center.
12.Salgado, G. F. J., Struts, A. V., Tanaka, K.,
Fujioka, N., Nakanishi, K., and Brown, M. F. (2004)
Biochemistry, 43, 12819-12828.
13.Parsell, E. (1971) Electricity and Magnetism
[in Russian], Nauka, Moscow.
14.Fliger, W. (1982) Structure and Dynamics of
Molecules [Russian translation], Mir, Moscow.
15.Shaitan, C. V. (1999) Soros Obrazovat.
Zh., No. 5, 8-13.
16.Baudry, J., Crouzy, S., Roux, B., and Smith, J.
C. (1999) Biophys. J., 76, 1909-1917.