Lipofuscin Granule Dynamics during Development of Age-Related Macular Degeneration
V. B. Saprunova1,2, D. I. Pilipenko1,2, A. V. Alexeevsky1, A. Zh. Fursova3, N. G. Kolosova3, and L. E. Bakeeva1,2*
1Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, 119991 Moscow, Russia; fax: (495) 939-0338; E-mail: fxb@belozersky.msu.ru2Mitoengineering Center, Lomonosov Moscow State University, 119991 Moscow, Russia; fax: (495) 939-5945; E-mail: info@skq-project.ru
3Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences, pr. Akademika Lavrentieva 10, 630090 Novosibirsk, Russia; fax: (383) 333-1278; E-mail: icg-adm@bionet.nsc.ru
* To whom correspondence should be addressed.
Received November 5, 2009
The pigment epithelium cell structure and therapeutic effect of antioxidant SkQ1, selectively penetrating into mitochondria from eye drops, were studied upon development in OXYS rats of age-related retinopathy as a model of macular degeneration. The characteristic dynamics and ultrastructural peculiarities of the layer of electron-dense cytoplasmic structures of the pigment epithelium apex part and incorporated lipofuscin granules were revealed. The therapy of OXYS animals for 68 days using 250 nM SkQ1 drops decreased the extent of development of age-related macular degeneration. Electron-microscopic investigation showed that SkQ1 prevented development of ultrastructural changes in the pigment epithelium characteristic of macular degeneration, the condition of which after therapy with SkQ1 drops corresponded to ultrastructure of pigment epithelium in Wistar rats of the same age having no symptoms of retinal damage. It is supposed that ultrastructural changes in the electron-dense layer upon development of age-related macular degeneration are indicative of disturbances in the optical cycle functioning, especially of disturbances in functioning of photoreceptor membranes.
KEY WORDS: electron microscopy, age dependent macular degeneration, pigment epithelium, mitochondria, lipofuscin granules, oxidative stress, SkQ1DOI: 10.1134/S0006297910020021
Abbreviations: A2E, N-retinol-N-retinylidene ethanolamine; ROS, reactive oxygen species; SkQ1, 10-(6′-plastoquinoyl)decyltriphenyl phosphonium.
Macular degeneration is the main reason for deterioration and loss of
vision in older people. The pathogenesis of this disease is poorly
studied. It is known that this pathology is accompanied by death of
photoreceptor cells and abundant accumulation of lipofuscin granules in
pigment epithelium. N-Retinol-N-retinylidene ethanolamine (A2E) is the
main fluorophore of lipofuscin. The accumulation of A2E becomes a key
factor resulting in development of age-related macular degeneration [1]. It was shown that in cell culture A2E is able to
induce cell apoptosis by damaging the outer mitochondrial membrane [1].
A unique model of aging of the organ of vision is the OXYS line of prematurely aging rats created in the Institute of Cytology and Genetics of Siberian Branch of the Russian Academy of Sciences (SB RAS). It was shown that in retinas of OXYS rats dystrophic alterations develop that correspond by clinical symptoms to human age-related macular degeneration [2]. Oxidative stress – disbalance of the systems of generation and detoxification of reactive oxygen species – is now considered as one of the main molecular mechanisms of pathogenesis of age-related macular degeneration. Antioxidants are able to prevent or delay premature aging symptoms in OXYS rats, and in this case their efficiency is due to the ability to prevent characteristic disturbances in mitochondrial functions [3, 4]. We have recently shown that the antioxidant 10-(6′-plastoquinoyl)decyltriphenyl phosphonium (SkQ1) in nanomolar concentrations prevents some consequences of accelerated aging in OXYS rats [5]. The distinguishing feature of SkQ1 is its ability to penetrate and accumulate in mitochondria – the main source of intracellular reactive oxygen species (ROS). An important advantage of SkQ1 is rapid reduction of its oxidized form by complex III of the mitochondrial respiratory chain, i.e. SkQ1 is an antioxidant of repeated action [5]. According to our data, the addition of SkQ1 to food completely prevented the development of cataract and retinopathy in OXYS rats up to two years of age [6, 7].
The goal of this work was to analyze the ultrastructure of pigment epithelium cells in OXYS and Wistar rats and therapeutic effect of the mitochondrion-directed antioxidant SkQ1 on development in OXYS rats of age-related macular degeneration.
MATERIALS AND METHODS
Animals. The OXYS line was bred in the Institute of Cytology and Genetics (SB RAS) from Wistar line by selection of animals susceptible to cataractogenic effect of galactose diet and inbreeding of highly susceptible animals [8]. After five cycles of inbreeding while feeding with galactose-rich food and the selection for cataract, the following rat generations spontaneously displayed cataract on galactose-free diet [9]. We now have the 90th generation of OXYS rats with spontaneously developed cataract and accelerated aging syndrome. This line of rats was called the OXYS line by the International Rat Genetic Nomenclature Committee [10].
This study was carried out on 30 male rats of OXYS line and 15 Wistar rats as controls. Nine-month-old OXYS and Wistar males were housed five animals to a cage. All animals got standard RK-120-1 diet (Laboratorsnab, Russia) and ad libitum water. The rats were kept at constant room temperature and natural illumination. All procedures were carried out on animals in accordance with the European Communities Council Directive No. 86/609/EES.
To study the effect of SkQ1 on development of retinopathy in OXYS rats, animals were divided to two groups, one of which was control and the other obtained SkQ1 in eye drops (250 nmol of SkQ1 were dissolved in 0.9% NaCl solution). Drops of SkQ1 (experimental group) or 0.9% NaCl (control group) were administered daily into eyes for two months beginning from the ninth day of life.
Ophthalmologic investigations of OXYS and Wistar rats were carried out using a Beta ophthalmoscope (Germany) equipped with a slit lamp after dilation by 1% tropicamide. The extent of choriocapillary and pigment epithelium cell dystrophy was estimated as follows: 1 unit meant the appearance of druses and other pathological alterations in the retina-related pigment epithelium and partial atrophy of the vascular membrane capillary layer; 2 units meant separation of pigment epithelium and retina-related neuroepithelium along with further atrophy of the vascular membrane capillary layer; 3 units correspond to neovascularization and separation of pigment epithelium and neuroepithelium cells and cicatrization.
Five days after the last eye examination, rats were decapitated and the effect of mitochondria-directed antioxidant SkQ1 on lipofuscin accumulation in pigment epithelium cells was studied.
Electron microscopy. Material for electron microscopy was fixed for 2 h at 4°C in 3% glutaraldehyde solution in buffer, pH 7.4. Then the material was additionally fixed with 1% osmium tetroxide solution for 1.5 h and dehydrated in solutions of increasing alcohol concentrations (70% ethanol saturated with uranyl acetate). The material was embedded in Epon-812 epoxide resin. Serial ultrathin sections were prepared on a Leica Ultracut ultramicrotome (Leica, Austria) and stained with lead according to Reynolds [11]. The preparations were examined and photographed in a HU-11B electron microscope (Hitachi, Japan).
Morphometric analysis. Counting was carried out on photographs covering section regions from 50 to 250 µm. A total of 22 pigment epithelium regions of 12 animals were studied. Amounts and section areas of electron-dense granules in pigment epithelium cells per µm were counted.
RESULTS
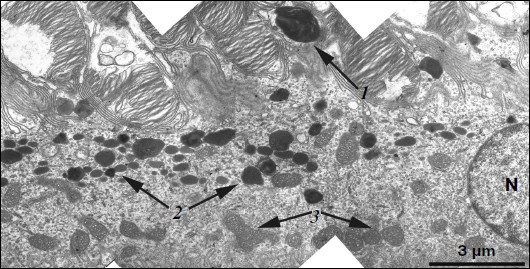
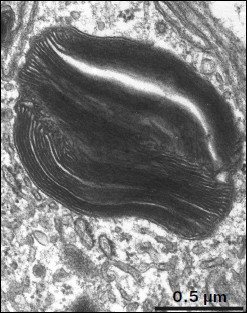
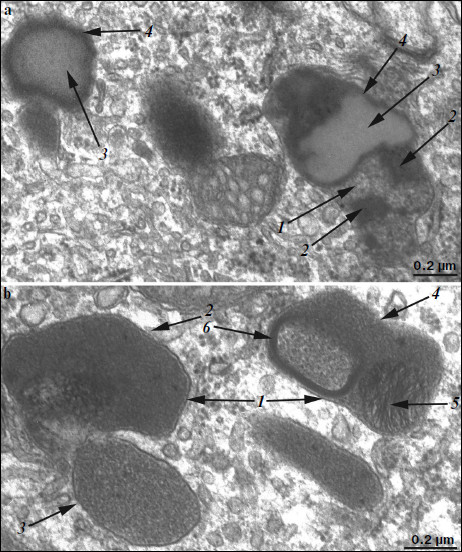
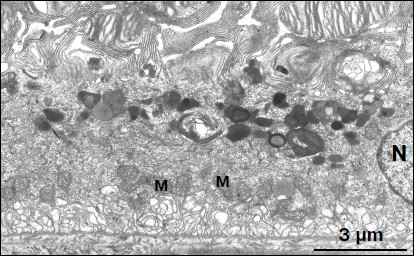
Ultrastructure of pigment epithelium cells. Pigment epithelium of Wistar cells (Fig. 1) consists of a layer of cells in the apical part of which there are fine strands of vertical extended cytoplasm offshoots surrounding rod outer segments. Phagosomes, debris of photoreceptor outer segments that underwent phagocytosis by pigment epithelium, sometimes can be seen in these cytoplasm offshoots near rod cell bases (Fig. 2). The characteristic ultrastructural feature of pigment epithelium is the presence in apical part of the cytoplasm of a layer of electron-dense structures (Fig. 1). As a rule, these structures are considered in the literature to be lipofuscin granules [12, 13]. We found in the apical part of pigment epithelium cells of 11-month-old Wistar rats practically continuous homogeneous layer of electron-dense structures – cytoplasmic inclusions (Fig. 1). Ultrastructural analysis of this layer showed that it contains morphologically extremely heterogeneous structures. First of all, these are lipofuscin granules. On the electron-microscopic photograph of the pigment epithelium region (Fig. 3a) arrows point to ultrastructure of two lipofuscin granules in the case of different section direction. It is seen that ultrastructure of lipofuscin granules corresponds to classical concepts. They can have round, oval, or polygonal shape depending on the section plane. Each granule is surrounded by a single-layer membrane contains moderately contrasting amorphous stroma substance (arrow 1) and aggregates of osmiophilic small granules (arrow 2). Oval osmiophobic regions or spherules (arrow 3) restricted by a layer of smallest particles of high electron density (arrow 4) can be seen within the amorphous substance. Besides, this layer of electron-dense structures includes large formations of variable size, shape, and internal arrangement. Figure 3b shows different structure of these formations. They are restricted by a single-layer membrane (arrow 1), contain granular stroma substance of different density in separate granules (for comparison arrows 2 and 3 point to granules of different density), and have heterogeneous ultrastructure along their length. Thus, arrow 4 in Fig. 3b points to a granule containing a region of fibrillar structure (arrow 5) as well as the close-type layers of concentric membranes restricting the space filled by the granular substance of electron density lower than that of stroma (arrow 6). We suppose that different ultrastructure of these formations is due to the fact that they are mutually transient structures, because various transient stages can be detected in their internal arrangement.
Fig. 1. Pigment epithelium region of 11-month-old Wistar rats. Arrows: 1) phagosomes; 2) the layer of electron-dense cytoplasmic inclusions; 3) mitochondria; N, cell nucleus.
Fig. 2. Phagosome ultrastructure at high magnification. Layers of photoreceptor membranes are clearly seen.
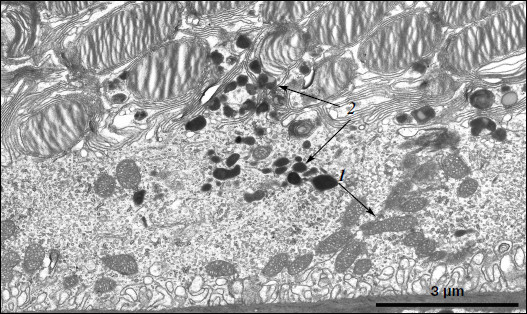
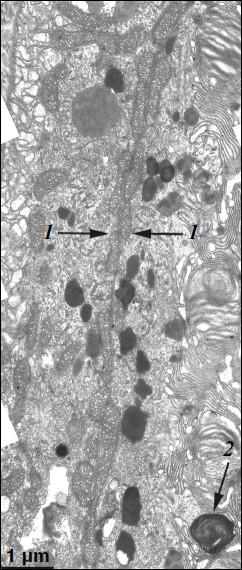
A different ultrastructural situation is specific of pigment epithelium cells of 11-month-old OXYS rats with retina damage symptoms of level 2 (Fig. 4). Main ultrastructural alterations are revealed in the cell apical part; first of all, this is the absence of a continuous layer of electron-dense inclusions as well as practically complete absence of phagosomes – debris of the photoreceptor outer segments phagocytized by pigment epithelium. Simultaneously, at the bases of offshoots of pigment epithelium cell cytoplasm, directed toward rod cells, aggregates of small electron-dense structures (mainly lipofuscin granules) are detected, which also fill in the offshoots themselves (Fig. 4).Fig. 3. Ultrastructural peculiarities of cytoplasmic inclusions – the layer of electron-dense structures. a) Lipofuscin granules. Arrows: 1) amorphous stroma substance; 2) aggregates of small osmiophilic granules; 3) osmiophobic regions or spherules; 4) electron-dense layer surrounding osmiophobic regions. b) Electron-dense structural formations. Arrows: 1) single-layer membrane; 2, 3) granules of different density; 4) a granule with heterogeneous ultrastructure; 5) a region of fibrillar structures; 6) closed-type layers of concentric membranes.
These alterations in the pigment epithelium ultrastructure are evidently indicative of disturbances in the visual cycle functioning. According to generally accepted concepts, during rhodopsin phototransformation in rod cells, in the norm trans-retinal complexed with phosphatidylethanolamine is transferred through photoreceptor membrane into the outer segment membrane and then further into the pigment epithelium cell [14-16]. Upon visual cycle disturbances, retinal, not removed in time from the disc photoreceptor membrane, is accumulated in the latter and then interacts with phosphatidylethanolamine, which results in formation in the membrane of bis-retinylidene phosphatidylethanolamine (A2E-PE), the precursor of bis-retinylidene ethanolamine (A2E), the main fluorophore of lipofuscin granules. Probably due to this, in the norm lipofuscin granules are revealed within electron-dense layer of cytoplasmic inclusions in the cytoplasm of apical part of pigment epithelium cells, whereas in OXYS rats lipofuscin granules are accumulated in the base of the pigment epithelium cell offshoots and fill in the space between rod cells, thus contributing to the development of further disturbances in the visual cycle functioning (it has been shown that lipofuscin granules are extremely phototoxic [1]). Practically complete absence of phagosomes also points to disturbances in functioning of photoreceptor membranes.Fig. 4. A region of pigment epithelium of eleven-month-old OXYS rat (retina damage of level 2). Arrows: 1) the border of two cells with adjacent cell restricting mitochondrial membranes; 2) the aggregate of small electron-dense structures at the bases of the pigment epithelium cytoplasm offshoots directed toward rod cells.
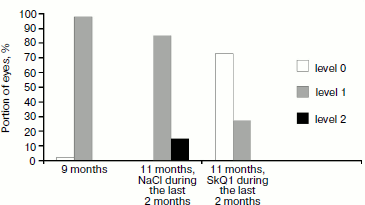
Effect of SkQ1 on development of retinopathy in OXYS rats. Examination of the rat fundus oculi before treatment with SkQ1 revealed the development of retinopathy in all OXYS rats. Only one eye (3%) of both (control and experimental) groups was free of pathology (Fig. 5). About 97% of eyes of OXYS rats from the control group have alterations corresponding to pre-disc-shaped stage of age-related macular degeneration (level 1). In the experimental group, 91% of eyes had alterations corresponding to pre-disc-shaped stage (level 1) and 16% eyes had alterations corresponding to the disc-shaped stage (level 2).
Comparison of the fundus oculi damage level in the control group of OXYS rats before and after treatment with 0.9% NaCl showed that clinical symptoms of age-related macular degeneration became more pronounced (p < 0.0005); as a result, in two months lesions in 9% eyes reached level 2, while in the other eyes the extent of macular degeneration corresponded to level 1.Fig. 5. Comparison of development of retinopathy in control and experimental OXYS rats before after treatment with eye drops containing 0.9% NaCl and 250 nM SkQ1.
Comparison of the extent of fundus oculi damage showed that SkQ1 decreased the development of age-related macular degeneration (p < 0.0005). Treatment with SkQ1 drops daily for 2 months decreased retinopathy symptoms and resulted in complete disappearance of retina pathology. Therapeutic effect was revealed in edema reduction and lowering in the amount and size of ischemic foci as well as in resorption of hemorrhages and pathological alterations in pigment epithelium pertaining to the retina. As a result, the number of the OXYS rat eyes free of pathological alterations reached 70%, while in the remaining 30% of eyes the extent of pathological alterations was estimated as level 1 (Fig. 5).
Effect of SkQ1 on ultrastructure of the OXYS rat pigment epithelium cells. To study the effect of SkQ1 on ultrastructure of OXYS rat pigment epithelium cells, five rats with maximal SkQ1 therapeutic effect (as shown by fundus oculi ophthalmoscopy, symptoms of pathological alterations decreased from level 2 to level 0) were selected for further investigations.
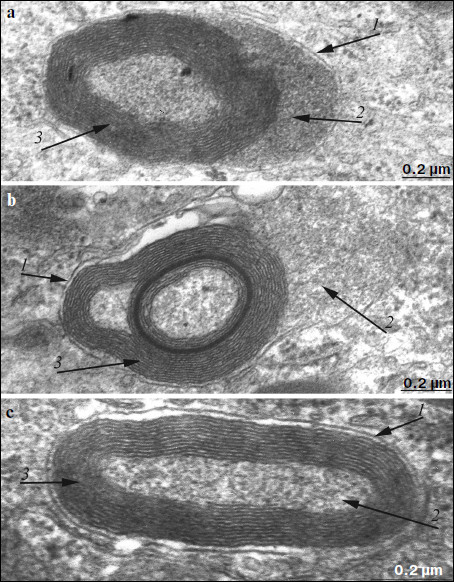
Electron microscopy showed that after treatment with SkQ1 drops, the ultrastructure of pigment epithelium cells changed significantly. An aggregate of numerous electron-dense structures looking as a strongly developed continuous layer, passing through all pigment epithelium cells, was revealed in apical cytoplasm of the latter (Fig. 6). It is also necessary to note that in separate regions of the pigment epithelium we detected the appearance of a second layer of cells with the strongly developed system of mitochondrial reticulum (Fig. 7). In this case, electron-dense structures were localized mainly in the upper layer of pigment epithelium cells. Our results show that, like in Wistar rats, morphology of these cytoplasmic inclusions is very diverse. They contain lipofuscin granules, but mainly this layer contains different structures. They are oval electron-dense structures filled by an electron-dense substance. We managed to find that separate such structures are mitochondria partially filled by electron-dense material. Besides, organelles of unusual ultrastructure are present in cytoplasmic inclusions. They are of round or spherical shape, surrounded by a single-layer membrane, its internal space filled by granulated matrix and tightly adjacent concentric membrane layers (Fig. 8). The origin and functional significance of these structures are still unknown.
Fig. 6. Region of pigment epithelium of 11-month-old OXYS rat treated with SkQ1 drops (250 nM). The layer of electron-dense cytoplasmic inclusions is clearly seen. N, cell nucleus; M, mitochondria.
Fig. 7. Region of pigment epithelium of 11-month-old OXYS rat treated with SkQ1 drops (250 nM). Arrows 1 point to the border between two layers of pigment epithelium cells with covering extended mitochondria. Arrow 2 points to a phagosome.
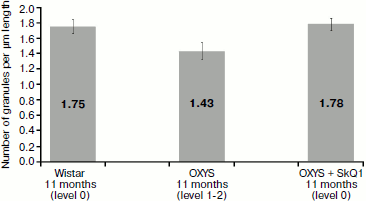
Morphometric analysis of electron-dense structures of pigment epithelium. As seen in Fig. 9, the number of granules in pigment epithelium in OXYS rats after treatment with SkQ1 was 1.78 granules per µm of length; in Wistar rats of the same age it was 1.75 granules per µm of epithelium length, whereas in OXYS rats without SkQ1 treatment the average number of granules was lower – 1.43 granules per µm.Fig. 8. Ultrastructure of separate electron-dense cytoplasmic inclusions in pigment epithelium of 11-month-old OXYS rats treated with SkQ1 drops (250 nM) under high magnification (a, b, c – different structural variants). Arrows: 1) restricting membranes; 2) granular matrix; 3) membrane concentric layers.
To compare the level estimation of retina condition with ultrastructure of pigment epithelium, the average density of granules was determined for all slices of the same rat material. It was found that in all four rats with retina damage symptoms of levels 1-2 the density did not exceed 1.5 granules per µm of Bruch’s membrane. At the same time, in seven out of eight rats with the level estimation of 0-0.5 unit, the density was over 1.5 granules per µm. Thus, the level estimations of retina condition reliably agree with our parameter characterizing ultrastructure of pigment epithelium (p < 0.01 according to precise Fisher criterion for tables of pairwise conjunction).Fig. 9. Total number of electron-dense granules in the rat pigment epithelium cells per µm epithelium length. All rats were 11-month-old. Levels characterizing the extent of retina damage are given in parentheses. After treatment with SkQ1, total number of intracellular granules in OXYS rat pigment epithelium is higher than that in OXYS rats of the same age not treated with SkQ1 (p < 0.02 according to precise Fisher criterion for tables of pairwise conjunction). Corresponding standard errors of the mean are indicated on columns.
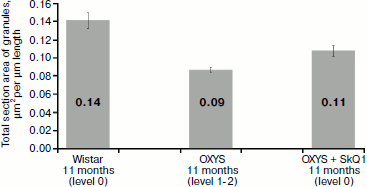
Another parameter calculated by us and characterizing ultrastructure of pigment epithelium is section area of electron-dense granules attributed to 1 µm of Bruch’s membrane. It correlates (Fig. 10) with the density of granules (coefficient of correlation is 0.7): in OXYS rats after treatment with SkQ1, total section area of electron-dense granules of pigment epithelium was 0.09 µm2 per µm of length, while in Wistar rats this value was 0.14 µm2 and in untreated OXYS rats it was 0.11 µm2.
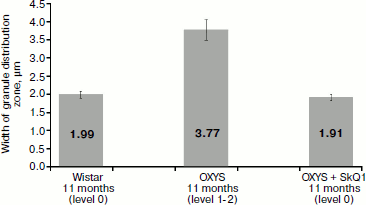
Measurements of the width of the zone of electron-dense granule distribution in pigment epithelium cells (Fig. 11) showed that in Wistar rats and in OXYS rats treated by SkQ1 granules were arranged in compact aggregates forming a continuous layer in the upper part of pigment epithelium cells, while in OXYS cells not treated with SkQ1 granules form no continuous layer: they are localized both in the upper part of pigment epithelium cells and in their cytoplasmic offshoots that twine around rod cells (Fig. 4).Fig. 10. Total section area of electron-dense granules in pigment epithelium cells per µm of epithelium length. All rats were 11-month-old. Levels characterizing the extent of retina damage are given in parentheses. After treatment with SkQ1, the total section area of granules in pigment epithelium cells of OXYS rats was higher than in OXYS rats of the same age not treated with SkQ1 (p < 0.02 according to precise Fisher’s criterion for tables of pairwise conjunction). Corresponding standard errors of the mean are indicated on columns.
The values correspond to those characterizing the extent of retina damage in OXYS rats treated with SkQ1, and in Wistar rats this value was level 0, while in 11-month-old OXYS rats not treated with SkQ1 damages of levels 1-2 were observed. Thus, these results agree with experimental observations. The reliability of correspondence of amounts of electron-dense granules and their section areas per µm of Bruch’s membrane length with the extent of retina damage levels was over 95% (by the precise Fisher’s criterion for tables of pairwise conjunction p < 0.05 for both correlations).Fig. 11. Width of the zone of electron-dense granule distribution in pigment epithelium cells. Corresponding standard errors of the mean are indicated on columns.
DISCUSSION
Our investigations have shown that development of age-related macular degeneration in OXYS rats is associated with alterations of pigment epithelium ultrastructure, in particular, with redistribution of localization as well as with excessive accumulation of lipofuscin granules in pigment epithelium regions adjacent to the rod cells. At the present time it is generally accepted that lipofuscin granules originate from lysosomes, in which dissociation products of rhodopsin membranes of the rod cell outer segments are accumulated. However, initially the possibility of the degenerating mitochondria transformation upon aging into lipofuscin granules was considered. Our observations show that both concepts can be valid. It is possible that the process of lysosome formation is specific of various tissues, and the appearance of lipofuscin granules is associated not only with aging, but it probably is of some more specific importance in intracellular metabolism. If previously lipofuscin granules were considered as harmless ballast, accumulated as far as aging proceeded, at the present time it has been shown that they are able to generate ROS in response to light [1].
Our results have shown that the mitochondria-directed antioxidant SkQ1 not only prevents progressive development of retinopathy in OXYS rats, but also decreases the severity of already development symptoms. It was found in the experiment on OXYS rats that only one eye of 60 had no clinical symptoms of retinopathy development. After therapy with SkQ1 drops for two months, symptoms of pathology were absent in 70% eyes of OXYS rats, while in control group further development of retinopathy was observed.
Our results have shown that SkQ1 partially reversed development of ultrastructural pathological alterations in pigment epithelium of OXYS rats. In pigment epithelium of OXYS rats after treatment with SkQ1, as well as in Wistar rats, there is a well-developed layer of electron-dense cytoplasmic inclusions (Figs. 1 and 3). Small electron-dense inclusions morphologically correspond to typical lysosomes filled with lipofuscin (like in liver cells), while structures analogous to large electron-dense formations of pigment epithelium are not known for other tissues. It can be supposed that structures comprising the layer of electron-dense cytoplasmic inclusions play an important role in maintenance of metabolism in outer segments of rod cells, providing in pigment epithelium of healthy animals for active processes of disintegration both of debris of outer segments of photoreceptors and of damaged cytoplasmic components. Found by us ultrastructural alterations in pigment epithelium of OXYS rats in response to SkQ1 confirm the now generally accepted concept that activation of free-radical processes plays one of key roles in development of age-related macular degeneration [1, 12-22].
REFERENCES
1.Ostrovsky, M. A. (2005) Uspekhi Biol. Khim.,
45, 173-204.
2.Kolosova, N. G., Lebedev, P. A., Fursova, A. Zh.,
Morozkova, T. S., and Gusarevich, O. G. (2003) Uspekhi
Gerontol., 12, 143-148.
3.Menshikova, E. B., Shabalina, I. G., Zenkov, N. K.,
and Kolosova, N. G. (2002) Byul. Eksp. Biol. Med.,
133, 207-210.
4.Kolosova, N. G., Shcheglova, T. V., Sergeeva, S.
V., and Loskutova, L. V. (2006) Neurobiol. Aging, 279,
1289-1297.
5.Skulachev, V. P. (2007) Biochemistry
(Moscow), 72, 1385-1396.
6.Neroev, V. V., Arkhipova, M. M., Bakeeva, L. E.,
Fursova, A. Zh., Grigoryan, E. N., Grishanova, A. Yu., Iomdina, E. N.,
Ivashchenko, Zh. N., Katargina, L. A., Kilina, O. V., Kolosova, N. G.,
Pilipenko, D. I., Kopenkin, E. P., Kovaleva, N. A., Novikova, Yu. P.,
Filippov, P. P., Robustova, O. V., Saprunova, V. B., Senin, I. I.,
Skulachev, M. V., Sotnikova, L. F., Tikhomirova, N. K., Stefanova, N.
A., Khoroshilova-Maslova, I. P., Tsapenko, I. V., Shchipanova, A. I.,
and Skulachev, V. P. (2008) Biochemistry (Moscow), 73,
1317-1328.
7.Skulachev, V. P., Anisimov, V. N., Antonenko, Y.
N., Bakeeva, L. E., Chernyak, B. V., Erichev, V. P., Filenko, O. F.,
Kalinina, N. I., Kapelko, V. I., Kolosova, N. G., Kopnin, B. P.,
Korshunova, G. A., Lichinitser, M. R., Obukhova, L. A., Pasyukova, E.
G., Pisarenko, O. I., Roginsky, V. A., Ruuge, E. K., Senin, I. I.,
Severina, I. I., Skulachev, M. V., Spivak, I. M., Tashlitsky, V. N.,
Tkachuk, V. A., Vyssokikh, M. Y., Yaguzhinsky, L. S., and Zorov, D. B.
(2009) Biochim. Biophys. Acta, 1787, 437-461.
8.Solov’eva, N. A., Morozkova, T. S., and
Salganik, R. I. (1975) Genetika, 18, 63-71.
9.Rumyantseva, Yu. V., Fursova, A. Zh., Fedoseeva, L.
A., and Kolosova, N. G. (2008) Biochemistry (Moscow), 73, 1176-1182.
10.Rat Genome Database (http://rgd.mcw.edu).
11.Reynolds, E. (1963) J. Cell. Biol.,
17, 208-212.
12.Imamura, Y., Noda, S., Hashizume, K., Shinoda,
K., Yamaguchi, M., Uchiyama, S., Shimizu, T., Mizushima, Y., Shirasawa,
T., and Tsubota, K. (2006) Proc. Natl. Acad. Sci. USA,
103, 11282-11287.
13.Winkler, B. S., Boulton, M. E., Gottsch, J. D.,
and Sternberg, P. (1999) Mol. Vis., 5, 32.
14.Sun, H., Molday, R. S., and Nathans, J. (1999)
J. Biol. Chem., 274, 8269-8281.
15.Kennedy, M. J., Lee, K. A., Niemi, G. A., Craven,
K. B., Garwin, G. G., Saari, J. C., and Hurley, J. B. (2001)
Neuron, 31, 87-101.
16.Sun, H., and Nathans, J. (2001) J. Bioenerg.
Biomembr., 33, 523-530.
17.Suzuki, M., Kamei, M., Itabe, H., Yoneda, K.,
Bando, H., Kme, N., and Tano, Y. (2007) Mol. Vis., 23,
772-778.
18.Beatty, S., Koh, H., Phil, M., Henson, D., and
Boulton, M. (2000) Surv. Ophthalmol., 45,
115-134.
19.Strunnikova, N., Zhang, C., Teichberg, D.,
Cousins, S. W., Baffi, J., Becker, K. G., and Csaky, K. G. (2004)
Invest. Ophthalmol. Vis. Sci., 45, 3767-3777.
20.Ames, B. N., Shigenaga, M. K., and Hagen, T. M.
(1993) Proc. Natl. Acad. Sci. USA, 90, 7915-7922.
21.Cai, J., Nelson, K. C., Wu, M., Sternberg, Jr.
P., and Jones, D. P. (2000) Prog. Retin. Eye Res., 19,
205-221.
22.Liang, F., and Godley, B. F. (2003) Exp. Eye
Res., 76, 397-403.