MINI-REVIEW: Creation of Catalytic Antibodies Metabolizing Organophosphate Compounds
I. N. Kurkova, I. V. Smirnov, A. A. Belogurov, Jr., N. A. Ponomarenko, and A. G. Gabibov*
Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, ul. Miklukho-Maklaya 16/10, 117997 Moscow, Russia; fax: (495) 330-6983; E-mail: kurkova.inna@gmail.com; smirnov@mx.ibch.ru; belogurov@mx.ibch.ru; natalie@mx.ibch.ru; gabibov@mx.ibch.ru* To whom correspondence should be addressed.
Received June 22, 2012; Revision received July 2, 2012
Development of new ways of creating catalytic antibodies possessing defined substrate specificity towards artificial substrates has important fundamental and practical aspects. Low immunogenicity combined with high stability of immunoglobulins in the blood stream makes abzymes potent remedies. A good example is the cocaine-hydrolyzing antibody that has successfully passed clinical trials. Creation of an effective antidote against organophosphate compounds, which are very toxic substances, is a very realistic goal. The most promising antidotes are based on cholinesterases. These antidotes are now expensive, and their production methods are inefficient. Recombinant antibodies are widely applied in clinics and have some advantage compared to enzymatic drugs. A new potential abzyme antidote will combine effective catalysis comparable to enzymes with high stability and the ability to switch on effector mechanisms specific for antibodies. Examples of abzymes metabolizing organophosphate substrates are discussed in this review.
KEY WORDS: catalytic antibodies, abzymes, organophosphate compounds, biocatalysisDOI: 10.1134/S0006297912100069
Abbreviations: abzyme, catalytic antibody; AChE, acetylcholinesterase; BChE, butyrylcholinesterase; OPT, organophosphorus toxins.
Organophosphate compounds were synthesized in the late 1930s as
insecticides. The high toxicity of these substances was then noticed by
the military. Although organophosphorus toxins (OPT) such as tabun,
sarin and soman were not widely used during World War II, large
quantities of these substances are still stored in military warehouses
in many countries and are in need of disposal [1].
There are documented cases of the use of OPT as chemical warfare agents
including the war between Iran and Iraq, military action against
Iraq’s Kurds [2] and the terrorist acts of
the Aum Shinrikyo sect in Matsumoto and Tokyo [3].
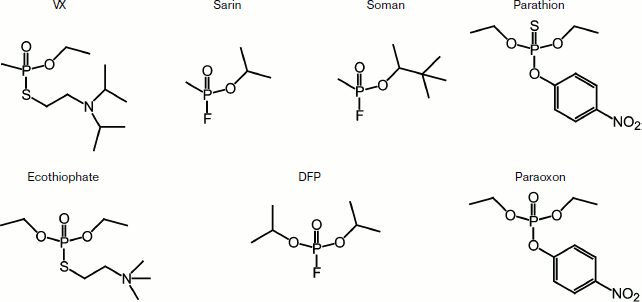
The chemical structures of some toxic organophosphorus compounds are
shown in Fig. 1.
All these substances have low molecular weights (from 140 to 300 Da), and the LD50 for these compounds are µg/kg, while for other toxins it is mg/kg. The mechanism of OPT action is based on the irreversible binding of the toxin to acetylcholinesterase (AChE, EC 3.1.1.7), which leads to the accumulation of acetylcholine at cholinergic synapses and consequently to the collapse of the peripheral and central nervous system, which affects the respiratory system of the body. Various data indicate that butyrylcholinesterase (BChE, EC 3.1.1.8) has a role in the prevention of cholinergic crisis through binding and removal of OPT from the body [4].Fig. 1. Chemical structures of some toxic organophosphorus compounds.
Availability of OPT pesticides in agriculture-based developing economies leads to frequent cases of poisoning and fatal intoxications with pesticides [5]. OPT poisoning is one of the most serious health problems, causing death of 200,000 people per year [6]. Furthermore, despite the fact that 185 states have acceded to the Convention on the Prohibition of Chemical Weapons, there is a threat of the use of nerve agents and other OPT during military and terrorist actions.
All the drugs currently used for the prevention and treatment of OPT poisoning require large doses and long-term drug treatment and cause some serious side effects [7, 8].
The development of protein-based drugs for detoxification of OPT is a promising area of modern toxicology. Currently, the most promising is the use of human BChE, paraoxonase and human serum albumin, as well as phosphotriesterases and oxidases from a variety of bacterial and eucaryotic sources [9].
In experiments on mice and nonhuman primates, it was shown that neutralization of organophosphorus cholinesterase inhibitors in the circulatory system by the prior administration of exogenous cholinesterases has a clear advantage over the conventional antidote as it provides protection against multiple lethal doses of highly toxic substances such as VX and soman without disrupting the animal’s life cycle. Studies in recent years have shown that the most promising preventive medication is the antidote butyrylcholinesterase (BChE) from human blood plasma [4]. Existing commercial systems for isolation of BChE are very expensive not sufficiently effective, and therefore require substantial improvement. Currently, recombinant antibodies are widely used as therapeutic agents and have several advantages over enzymes. A potential antidote created on the basis of a catalytic antibody (abzyme) could combine the properties of the enzyme catalyst with low immunogenicity, long period of circulation in the bloodstream and effective mechanisms for removal from the body in the antigen–antibody complex, which are characteristic of the immunoglobulin molecule. Despite the fact that the catalytic efficiency of abzymes is much less than that of evolutionarily optimized enzyme biocatalysts, the creation of “catalytic” vaccine, i.e. catalytic antibodies capable of neutralizing OPT is a very important task. In this review we present examples of catalytic antibodies that metabolize organophosphorus substrates.
ANTIBODIES AS ARTIFICIAL ENZYMES
For development of catalytic antibodies, it is necessary to consider a number of restrictions that are imposed by the nature of antibodies on the functionality of these biocatalysts in comparison with enzymes. Existing catalytic antibodies have much lower natural catalytic efficiency than enzymes. It now seems clear that catalysis by stabilizing a transition state of a reaction can provide only a moderate acceleration of the reaction. This is because the ratio of the rate constants of catalyzed and uncatalyzed reactions is determined by the ratio of dissociation constants of an antibody with the reaction substrate and with an analog of its transition state, and this ratio is unlikely to be high [10, 11]. However, the reactions catalyzed by natural enzymes are found to include additional mechanisms such as destabilization of the initial substrate, the convergence of the catalytically active groups and the attacked chemical bond in the active site, dynamics of the active site and several other mechanisms. Since the selection of clones of B-lymphocytes that produce antibodies to an introduced antigen, which is usually limited by the achievement of a dissociation constant of 10–6-10–9 M, it seems clear that the development of abzymes possessing properties of enzymes cannot be reduced to the immunization of animals and screening of the resulting hybridoma clones.
The immunoglobulin nature of antibodies provides them with a number of advantages over enzymes for potential use as medicines. These include low immunogenicity, prolonged half-life of antibodies in the bloodstream, effective mechanisms for removing them from the body as a complex with the antigen, as well as extensive experience with drugs based on antibodies in medical practice. There are more than 30 drugs based on antibodies approved for clinical use, and it is projected that by 2011 the sales of these products will reach 21 million USD, which corresponds to the second place after vaccines [12]. The unique modular structure of immunoglobulin molecules allows the use of genetic engineering technology to modify their functional properties (e.g. molecular size, specificity, affinity, valence, immunogenicity, effector properties, and the possibility of making fusion proteins) in accordance with the requirements of clinical practice [12]. Even the low rate of catalysis by abzymes can be an advantage in cases when a therapeutic agent is needed to have not quick but lasting effect.
Strategies of abzyme development have been recently expanded to functional selection of combinatorial libraries of diverse protein domains. Ligands that can interact with the active site of enzymes, including analogs of transition states, conformational inhibitors and suicidal substrates/inhibitors, have been used for affinity and covalent selection of functional enzyme molecules.
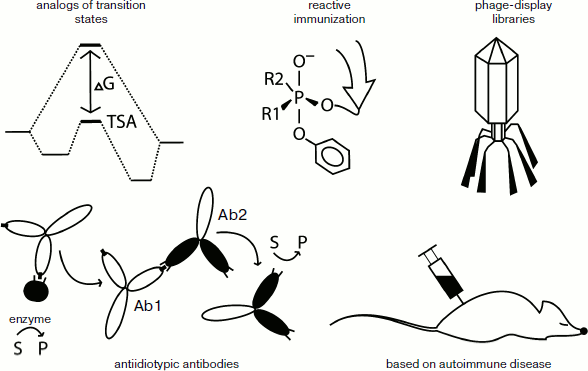
Methods for producing catalytic antibodies. There are several approaches currently being used to obtain artificial catalytic antibodies: immunizing animals by analogs of transition states; preparing antiidiotypic antibodies to the active site of already known enzymes; reactive immunization; induction of autoimmune diseases in animal models; screening of phage-display libraries of immunoglobulin genes (Fig. 2).
Among these approaches, the most interesting from the point of view of fundamental enzymology are methods based on the mechanism of the designed functioning of the abzyme. These include the preparation of abzymes by immunizing animals with analogs of transition states, as well as the selection of phage-display libraries of immunoglobulin genes.
We should also mention the use of phosphonates for the induction of hydrolytic abzymes. Some phosphonates are effective inhibitors of esterases and proteases due to their high similarity with structures based on tetrahedral carbon, which are seen as a transitional state of hydrolytic reactions [13]. Thus, the phosphonates are analogs of the transition states for hydrolytic reactions and analogs of OPT in the mechanism of action on cholinesterase. Using various phosphonates based haptens, a large number of antibodies capable of hydrolyzing esters [14, 15], carbonates [16], some amides [17] and OPT [18] have been obtained. The structure and properties of some of these will be discussed in detail below.Fig. 2. Methods for induction of catalytic antibodies.
IMMUNIZATION WITH TRANSITION STATE ANALOGS
Many important bodily functions require highly specific affinity interactions. Indeed, the formation of high affinity complexes (DNA–protein, hormone–receptor, antigen–antibody, transition state analog of an enzymatic reaction–enzyme) involves hydrogen bonds, van der Waals, hydrophobic interactions and ionic bonds. According to the concept of Jenks [19], the enzyme–transition state analog interaction can mimic antigen–antibody binding. The principle of stabilizing the transition state of the reaction provided the first successes in the creation of catalytic antibodies, and this has been used in many subsequent works. General principles for design of catalytically active antibodies were formulated in 1990 in a review by Shokat and Schultz [20]: catalysis carried out by abzyme based on the known principles of enzymatic catalysis and involving stabilizing the transition state, general acid–base catalysis, nucleophilic and electrophilic types of catalysis, as well as convergence effects.
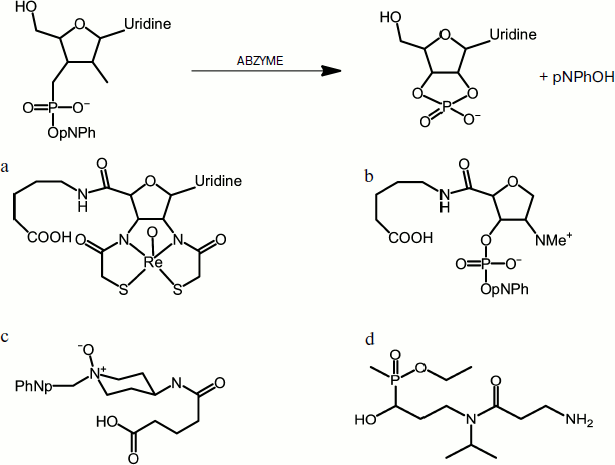
Janda et al. used two strategies to generate antibodies that are capable of hydrolyzing phosphodiester bonds: a chelate compound of oxyrhenium(V) was used as a transition state analog [21] and the approach of “bait and switch” [22]. In the first case, the authors showed that a stable pentacoordinated oxyrhenium(V), which mimics the transition state of phosphodiester bond hydrolysis (trigonal bipyramid), is capable of producing antibodies, which cleave uridine 3′-(p-nitrophenyl) phosphate with a rate constant kcat 0.09 min–1 (Fig. 3a). The more efficient method of “bait and switch” is based on obtaining antibodies to hapten that possess a charged chemical group. In this case, immunoglobulins were produced that carry the opposite charge in the active site. Thus, it was suggested that the introduction of a dimethylamino group in position 2 for 2-deoxyribose might lead to the induction of a negatively charged amino acid residue in the active site. This in turn will activate the 2-hydroxyl group for the attack of the phosphodiester bond (Fig. 3b). The antibodies obtained in this way hydrolyzed 3-(p-nitrophenylphospho)-1-deoxyribitol with 1650-fold acceleration of rate constant to 0.44 min–1.
Fig. 3. Use of analogs of the transition state of hydrolysis of a phosphodiester bond to produce catalytic antibodies. A scheme of one catalyzed reaction (upper panel) and examples of reaction transition state analogs used in different studies (a-d).
ABZYME
Another method proposed by Janda’s group is immunization with amine oxides (Fig. 3c), which yielded antibodies that were capable of hydrolyzing paraoxon (kcat 1.3⋅10–3 min–1) and similar phosphotriesters [23].
Another striking example of catalytic antibodies capable of hydrolyzing organophosphate toxins is the work by Vayron et al. [24]. They demonstrated that antibodies can be effective agents neutralizing organophosphorus chemical warfare agents, in particular methylphosphonothioate (gas VX) (Fig. 1). As a primary immunogen, the hapten methyl-α-hydroxyphosphinate was used; it is an analog of the transitional state that mimics a stage of the attack of hydroxide ion on the phosphorus atom during the hydrolysis of VX (Fig. 3d). The resulting polyclonal catalytic antibodies were capable of hydrolyzing methylphosphonothioate at a moderate rate. Using the modified hapten phenyl-α-hydroxyphosphinate, monoclonal abzymes were obtained that catalyzed the decay of a compound VX analog, phenylphosphonothioate, with 14,400-fold acceleration to the rate constant of 0.36 M–1·min–1.
Thus, the production antibodies to transition states analogs is a very effective way to obtain abzymes. The resulting antibodies had specific antigen-binding sites, which were required for binding of the transition state of a reaction as demonstrated by X-ray analysis. However, this method is not based on the principle of selection of the catalytic site possessing the activated amino acid residue or residues. Furthermore, a very strong binding of the transition state may interfere with catalysis. So, in a series of papers attempting to improve the properties of catalytic antibodies by screening libraries of mutants for binding to analogs of transition states [25, 26], it was shown that the antibodies that have the greatest affinity for the transition state analog showed lower catalytic activity than the parent antibody. Weakening of the ability to bind the transition state analog in some cases led to an increase in catalytic activity by 2-fold compared with the parent abzymes [25].
To avoid these shortcomings, a group of “reactive” methods of development of catalytic antibodies that combined reactive immunization and reactive selection was developed.
Development of abzymes by reactive immunization. An interesting alternative to the traditional method for producing reactive immunization abzymes was described in 1995 by the group of R. Lerner [27]. This method is based on the immunization of animals with hapten-based covalent inhibitors of serine hydrolases. It was assumed that these antigens would form a covalent complex with an antibody having a suitable active site structure and thus stimulate the proliferation of B-cell clones. The efficiency of the method was demonstrated by immunizing mice with a hapten containing an organophosphorus diester reactive group (i.e. dialkylphosphonate), and it exhibited a high proportion of clones of hybridomas secreting esterolytic antibodies. The obvious advantage of reaction immunization over the immunization with transition state analog is the possibility to induce abzymes employing a certain mechanism of catalysis.
One example of the use of the reactive immunization to obtain a catalyst is the work of Ponomarenko et al., where they used immunization with peptidylphosphonate (LAEEE-VPhos), the peptide part of which is represented by the fragment of HIV-1 glycoprotein gp120. The resulting antibodies E11 and E6 were able to specifically bind a peptide fragment of the “reactive” hapten and covalently interact with its phosphonate component. In addition, the abzymes catalyze the hydrolysis of low molecular weight substrate LAEEEV-MCA (kcat (1.1 ± 0.5)·10–3 min–1, Km 53 ± 14 µM for antibody E6 and kcat (3.2 ± 0.7)·10–4 min–1, Km 48 ± 11 µM for antibody E11) [28].
Reactive selection based on phage display. All the screening and selection methodologies are based on the relationship between the gene encoding the enzyme and the functional activity of the expressed protein. Phage display is one of the best methods known to date, which can be used to establish a functional link between the protein and the gene that encodes it [29-31]. Most of the artificial enzymes described to date have been created using for the cloning gene III [32]. As a result, the enzyme and protein pIII are combined into a single polypeptide by a peptide linker. In such designs all copies of pIII (3-5) should carry a cloned enzyme. However, in practice, due to proteolytic degradation of the enzyme or linker, the number of copies is reduced. In principle, selection methods can be divided into two broad categories: direct selection methods that use substrates and indirect selection methods that use inhibitors.
The indirect method of selection is selection for irreversible inhibitors. With this approach, libraries of enzyme variants are incubated with a certain concentration of biotinylated inhibitor under kinetic control by the experimenter [32, 33]. Phage particles that carry the most active enzymes are the first to react and become covalently bound to biotinylated molecules (become “labeled”). In the next stage, “labeled” phage particles are selected using streptavidin or its analogs. The bound phages are usually eluted either by proteolytic cleavage of phage particles or by reducing disulfide bonds that were introduced into the biotinylated agent.
Irreversible inhibitors of serine proteases have also been used to obtain new proteolytic enzymes [34, 35]. Thus, for example, Paul et al. [35] used biotinylated phosphonates for the selection of proteolytic antibodies from a library of antibody genes derived from lymphocytes of patients with systemic lupus erythematosus (SLE), as well they used these to immunize mice. The paper shows the possibility of using phosphonates (as di- and monoesters) to search for antibodies with proteolytic activity, but this study has not been followed up.
The method of reactive selection was used in a joint work of the laboratory of biocatalysis (Institute of Bioorganic Chemistry, Russian Academy of Sciences) and Prof. A. Tramontano (University of California, Davis, California). By the selection of semi-synthetic libraries of variable fragments of human immunoglobulin genes with p-nitrophenyl 8-methyl-8-azabicyclo[3.2.1]octane (phenylphosphonate X), recombinant single-chain antibodies were obtained that covalently interacted with irreversible inhibitors of serine hydrolases. For the selected clones, the amino acid residues that were modified by phosphonate were identified. In addition, it was found that the selected recombinant antibodies were capable of hydrolyzing the amide bond. These single-chain antibodies can be considered as an example of the simplest devices with the active site of enzymes and could be used as a template for a new artificial enzymes production through directed evolution [13].
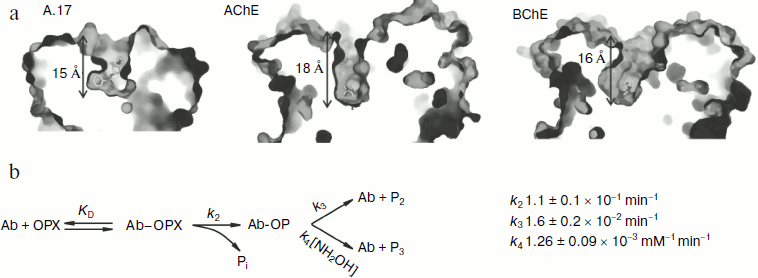
In the continuation of this work on the basis of sequences of variable domains of single-chain antibody A.17, a full-size antibody was designed and expressed in CHO-K1 cells. It was shown that the full-size antibody is capable of covalent binding to phenylphosphonate (X). In addition, the reaction of A.17 with a biotinylated derivative of phosphonate X (Bt-X) is inhibited by diisopropylfluorophosphate (DFP), 4-(2-aminoethyl) benzylsulfonyl fluoride (AEBSF) and by the insecticide paraoxon. Structural studies demonstrated that abzyme A.17 has a deep antigen-binding pocket, at the bottom of which is located the catalytic residue TyrL37. Such a deep active site is not typical for catalytic antibodies and it is more characteristic for catalytic sites of cholinesterases, in particular, butyrylcholinesterase (Fig. 4a).
Based on structural analysis of a Fab fragment of the catalytic antibody A.17 and its derivative modified by a phosphonate X, mutagenesis of several amino acid residues of the antibody was carried out to identify their role in the catalytic activity of the abzyme. It was possible to show that the substitution of residues involved in stabilizing CDR-H3 affect the reaction rate of phosphonylation of the antibodies by phosphonate X and has little effect on the stage of substrate binding. Structural analysis and study of thermodynamics and fast kinetics revealed that the interaction of A.17 with phosphonate X takes place by the induced fit mechanism. In addition, it was found that hydrolysis of the pesticide paraoxon by A.17 antibody passes through a stage of formation of phosphotyrosine covalent intermediate with amino acid residue Y-L37. The dephosphorylation stage is rate limiting (Fig. 4b).Fig. 4. Comparison of depths of cavities of the active centers of different types of biocatalysts. a) A.17, a catalytic antibody; AChE, acetylcholinesterase; BChE, butyrylcholinesterase. In each case the depth of the active center corresponds to the height of the pyramid inscribed into the cavity of the active site in such a way that its vertex coincides with the reaction residue, and the base lies in the plane drawn through the three amino acid residues next to the entrance of the active site of the biocatalyst. b) Kinetic scheme of hydrolysis of paraoxon by antibody A.17.
Ability to interact with organophosphate molecules as a “byproduct”. In some cases, antibodies that were obtained by other methods also have the ability to metabolize organophosphorus molecules.
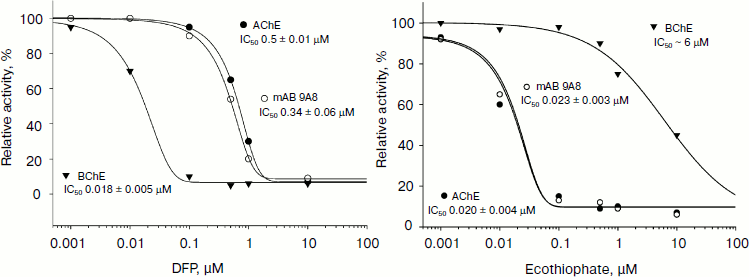
In [36] and later in Kolesnikov et al. [37] an antiidiotypic approach to generate antibodies to human acetylcholinesterase was used. Thus, the IgM 9A8 antibody, which was obtained as idiotypic antibody AE2, inhibited the activity of acetylcholinesterase, and it also possessed the ability to interact with paraoxon and ecothiophate with activity comparable to that of the native enzymes (Fig. 5). It is interesting to note that despite the absence of any structural homology between the enzyme and abzymes, both amino acid sequence and 3D model, they show similar substrate specificity. From the combination of mass spectrometric studies and analysis of the model structure, the authors suggested the presence in the catalytic active site of the abzyme a Ser-His dyad, which explains the presence of catalytic antibody activity similar to acetylcholinesterase.
The use of organophosphorus molecules in the creation of catalytic antibodies is very common. In most cases, phosphonates are used as analogs of the transition state, and the resulting antibodies are capable of only reversible binding of the hapten. In the case of the reactive methods of abzyme production, catalytic antibodies usually possess the ability to be covalently modified by phosphonate like serine hydrolases. In papers of Gabibov et al. it was demonstrated that this in turn may indicate the potential ability of these catalytic antibodies to metabolize OPT. For example, antibody A.17 is capable of catalyzing the hydrolysis of the pesticide paraoxon at a low rate.Fig. 5. Analysis of interaction of antiidiotypic antibody 9A8 with organophosphorus inhibitors diisopropylfluorophosphate (DFP) (left) and ecothiophate (right).
It should be noted that considerable progress made in abzymology in such a short period of time indicates the vast prospects of this field of science. However, effective catalysis of the reactions with high-energy transition states realized by the enzymes through a complex set of mechanisms is not fully implemented for antibodies.
REFERENCES
1.Greenfield, R. A., Brown, B. R., Hutchins, J. B.,
Iandolo, J. J., Jackson, R., Slater, L. N., and Bronze, M. S. (2002)
Am. J. Med. Sci., 323, 326-340.
2.Macilwain, C. (1993) Nature, 363,
3.
3.Tu, A. T. (1996) J. Mass. Spectrom. Soc.
Jpn., 44, 293-320.
4.Lenz, D. E., Yeung, D., Smith, J. R., Sweeney, R.
E., Lumley, L. A., and Cerasoli, D. M. (2007) Toxicology,
233, 31-39.
5.Kwong, T. C. (2002) Ther. Drug Monit.,
24, 144-149.
6.Eddleston, M., Buckley, N. A., Eyer, P., and
Dawson, A. H. (2008) Lancet, 371, 597-607.
7.Wadia, R. S. (2003) J. Crit. Care Med.,
7, 85-87.
8.Eyer, P., Eddleston, M., Thiermann, H., Worek, F.,
and Buckley, N. A. (2008) Br. J. Clin. Pharmacol., 66,
451-452.
9.Masson, P., and Rochu, D. (2009) Acta
Natura, 1, 68-78.
10.Hilvert, D. (2000) Annu. Rev. Biochem.,
69, 751-793.
11.Stewart, J. D., and Benkovic, S. J. (1995)
Nature, 375, 388-391.
12.Deyev, S. M., and Lebedenko, E. N. (2009) Acta
Natura, 1, 32-50.
13.Reshetnyak, A. V., Armentano, M. F., Ponomarenko,
N. A., Vizzuso, D., Durova, O. M., Ziganshin, R., Serebryakova, M.,
Govorun, V., Gololobov, G., Morse, H. C., 3rd, Friboulet, A., Makker,
S. P., Gabibov, A. G., and Tramontano, A. (2007) J. Am. Chem.
Soc., 129, 16175-16182.
14.Pollack, S. J., Jacobs, J. W., and Schultz, P. G.
(1986) Science, 234, 1570-1573.
15.Tawfik, D. S., Lindner, A. B., Chap, R., Eshhar,
Z., and Green, B. S. (1997) Eur. J. Biochem., 244,
619-626.
16.Suzuki, H., Mukouyama, E. B., Wada, C.,
Kawamura-Konishi, Y., Wada, Y., and Ono, M. (1998) J. Protein
Chem., 17, 273-278.
17.Janda, K. D., Schloeder, D., Benkovic, S. J., and
Lerner, R. A. (1988) Science, 241, 1188-1191.
18.Smirnov, I., Carletti, E., Kurkova, I., Nachon,
F., Nicolet, Y., Mitkevich, V. A., Debat, H., Avalle, B., Belogurov, A.
A., Jr., Kuznetsov, N., Reshetnyak, A., Masson, P., Tonevitsky, A. G.,
Ponomarenko, N., Makarov, A. A., Friboulet, A., Tramontano, A., and
Gabibov, A. (2011) Proc. Natl. Acad. Sci. USA, 108,
15954-15959.
19.Jenks, W. P. (1969) Catalysis in Chemistry and
Enzymology, McGraw-Hill, New York.
20.Shokat, K. M., and Schultz, P. G. (1990) Annu.
Rev. Immunol., 8, 335-363.
21.Weiner, D., Wiemann, T., Wolfe, M., Wentworth,
P., Jr., and Janda, K. (1997) J. Am. Chem. Soc., 119,
4088-4089.
22.Wentworth, P., Jr., Liu, Y., Wentworth, A. D.,
Fan, P., Foley, M. J., and Janda, K. D. (1998) Proc. Natl. Acad.
Sci. USA, 95, 5971-5975.
23.Gao, C., Lavey, B. J., Lo, C. H., Datta, A.,
Wentworth, P., Jr., and Janda, K. D. (1998) J. Am. Chem. Soc.,
120, 2211-2217.
24.Vayron, P., Renard, P. Y., Taran, F., Creminon,
C., Frobert, Y., Grassi, J., and Mioskowski, C. (2000) Proc. Natl.
Acad. Sci. USA, 97, 7058-7063.
25.Baca, M., Scanlan, T. S., Stephenson, R. C., and
Wells, J. A. (1997) Proc. Natl. Acad. Sci. USA, 94,
10063-10068.
26.Takahashi, N., Kakinuma, H., Liu, L., Nishi, Y.,
and Fujii, I. (2001) Nat. Biotechnol., 19, 563-567.
27.Lerner, R. A., and Barbas, C. F., 3rd. (1996)
Acta Chem. Scand., 50, 672-678.
28.Durova, O. M., Vorobiev, I. I., Smirnov, I. V.,
Reshetnyak, A. V., Telegin, G. B., Shamborant, O. G., Orlova, N. A.,
Genkin, D. D., Bacon, A., Ponomarenko, N. A., Friboulet, A., and
Gabibov, A. G. (2009) Mol. Immunol., 47, 87-95.
29.Hoess, R. H. (2001) Chem. Rev.,
101, 3205-3218.
30.Smith, G. P., and Petrenko, V. A. (1997) Chem.
Rev., 97, 391-410.
31.Winter, G., Griffiths, A. D., Hawkins, R. E., and
Hoogenboom, H. R. (1994) Annu. Rev. Immunol., 12,
433-455.
32.Fernandez-Gacio, A., Uguen, M., and Fastrez, J.
(2003) Trends Biotechnol., 21, 408-414.
33.Soumillion, P., Jespers, L., Bouchet, M.,
Marchand-Brynaert, J., Sartiaux, P., and Fastrez, J. (1994) Appl.
Biochem. Biotechnol., 47, 175-189; discussion 189-190.
34.Legendre, D., Laraki, N., Graslund, T., Bjornvad,
M. E., Bouchet, M., Nygren, P. A., Borchert, T. V., and Fastrez, J.
(2000) J. Mol. Biol., 296, 87-102.
35.Paul, S., Tramontano, A., Gololobov, G., Zhou, Y.
X., Taguchi, H., Karle, S., Nishiyama, Y., Planque, S., and George, S.
(2001) J. Biol. Chem., 276, 28314-28320.
36.Izadyar, L., Friboulet, A., Remy, M. H., Roseto,
A., and Thomas, D. (1993) Proc. Natl. Acad. Sci. USA, 90,
8876-8880.
37.Kolesnikov, A. V., Kozyr, A. V., Alexandrova, E.
S., Koralewski, F., Demin, A. V., Titov, M. I., Avalle, B., Tramontano,
A., Paul, S., Thomas, D., Gabibov, A. G., and Friboulet, A. (2000)
Proc. Natl. Acad. Sci. USA, 97, 13526-13531.