REVIEW: Glycobiology of Human Milk
D. S. Newburg
Program in Glycobiology, Department of Biology, 140 Commonwealth Avenue, Boston College, Chestnut Hill, MA 02467, USA; fax: 617-552-2011; E-mail: david.newburg@bc.edu
Received March 7, 2013; Revision received March 9, 2013
Glycans are characteristic components of milk, and each species has unique patterns of specific carbohydrates. Human milk is unusually rich in glycans, with the major components being lactose and oligosaccharides, representing approximately 6.8 and 1% of the milk, respectively. Other sources of glycans in human milk include monosaccharides, mucins, glycosaminoglycans, glycoproteins, glycopeptides, and glycolipids. In human milk, the presence and patterns of these glycans vary depending upon the stage of lactation and the maternal genes and their genetic polymorphisms that control glycosyl transferases. The synthesis of milk glycans utilizes a significant portion of the metabolic energy that the mother expends when producing her milk, but other than lactose, these glycans contribute little to the nutritional needs of the infant. The data herein support several functions. 1) Many human milk glycans inhibit pathogens from binding to the intestinal mucosa. 2) Human milk glycans attenuate inflammation. 3) Glycans also directly stimulate the growth of beneficial (mutualist) bacteria of the microbiota (formerly considered commensal microflora of the intestine); these mutualists and their fermentation products can, in turn, (a) inhibit pathogens, (b) modulate signaling and inflammation, and (c) the fermentation products can be absorbed and utilized as a source of dietary calories. These functions can help direct and support intestinal postnatal growth, development, and ontogeny of colonization. The many functions of the milk glycans may synergistically protect infants from disease. Hence, human milk glycans and their homologs may serve as novel prophylactic or therapeutic agents for a diverse range of deleterious conditions.
KEY WORDS: human milk glycans, lactose, oligosaccharides, glycoproteins, mucins, glycosaminoglycans, glycolipidDOI: 10.1134/S0006297913070092
Abbreviations: GAG, glycosaminoglycan; HMOS, human milk oligosaccharides; LDFH-I, lactodifucohexaose-1; LDFT, lactodifucotetraose; Neu5Ac, N-acetylneuraminic acid; Neu5Gc, N-glycolylneuraminic acid; 2′-FL, 2′-fucosyllactose; 3-FL, 3-fucosyllactose.
Dedicated to my mentor and friend, Dr. Robert H. McCluer
(April 13, 1928 - September 8, 2005)
The class Mammalia is named for the unique ability of its females to provide milk to their infants via their mammary secretions. Milk produced by the mammary gland is the first food of all mammals. The platypus, an egg-laying mammal (monotreme) that is considered representative of the evolutionary transition to maternal milk production, produces an exudate from a mammary skin patch of modified sweat glands and hair follicles that the infant licks; the exudate primarily contains molecules that protect the infant from disease. The marsupial mammals, remnants of a once more widely dispersed and numerous infraclass, provide milk to their offspring from a teat in the pouch where the young are kept. As the offspring matures, the milk composition changes from a fluid rich in protective molecules, including a large amount of unique milk oligosaccharides, to a richer milk containing lactose and other key nutrients for growth.
It is worth noting that milk oligosaccharides are very poorly digested or absorbed, consistent with functions that are mostly confined to the lumen of the gut, such as antipathogenic (competitive inhibitor of a pathogen receptor), immunomodulatory (attenuating excessive inflammation), or prebiotic (promoting colonization by, and growth of, mutualist symbionts) activity [1]. The eutherians, placental mammals, deliver their offspring at a more mature stage than the other mammals, and their milk is rich in nutrients. However, their initial mammary secretions, colostrum, contain mainly protective molecules, while true milk contains significant amounts of nutrients such as casein and lactose, which normally appear a few to several days after parturition.
For humans, whose offspring continue their growth and development for a significant portion of their lives, the extended provision of milk is a pronounced feature; indeed, secondary sexual characteristics that suggest the potential of ample provision of milk are highly valued by prospective mates. In human infants, the natural period of exclusive breastfeeding coincides with the most rapid period of brain development. Therefore, it is notable that humans have a checkered 5000-year history of periodically entrusting the growth of our infants to human milk substitutes, especially during periods of rapid social change. This occurs despite recorded medical observations throughout the ages of negative consequences to the health of the infant when fed artificially.
Over the past century, the advance of pediatrics as a medical specialty was accompanied by increased research interest in human milk. However, we still know less about milk than most other aspects of our biology, even with the recognition that milk is naturally the exclusive source of nutrients at a critical time for infant growth, especially brain growth and development. Researchers of human milk, including glycobiologists, pediatricians, dairy researchers, and others, have defined and are still defining human milk as a very complex mixture. With more powerful tools for defining glycans, the unusually high level of glycosylation in milk components and their bioactivity are now becoming more fully appreciated. Much of the biological activity seems to be directed at protecting the infant from pathogens [1].
The concentrations of glycans in human milk can vary among individuals, over the course of lactation, and from the beginning to the end of a feeding session, but typically do not vary between breasts. The first milk of a given feed, the foremilk, has less fat than the last milk of a feed, the hindmilk, and thus the glycans that are found in the aqueous portion of milk tend to decrease in concentration as the feed progresses and fat increases. Conversely, those associated with the milk fat globule may increase. These changes are often masked by the larger amount of error intrinsic to many types of analyses. The combination of biological variation, sampling differences, and components of milk that interfere with many of the common analytical techniques could account for the high variation in reported values of glycan concentrations in human milk. However, new analytical methods and carefully controlled studies on large populations suggest that the true biological variability of some human milk glycans is indeed quite high, and that their expression in a population and across populations can be quite diverse.
The large number of distinct types of glycans present in milk is daunting. Almost all of the milk proteins are glycosylated, many quite extensively, and milk also contains large quantities of mucins and glycosaminoglycans. The major glycans in milk are lactose and oligosaccharides, with relatively little free monosaccharide. The free monosaccharides vary between individuals, and within the same individual depending on the stage of lactation and other factors.
MONOSACCHARIDES
Glucose is present in the earliest milk, colostrum, at 0.03, 0.06, and 0.16 g/liter during days 1, 2, and 3 of lactation, respectively. Glucose in transitional milk (days 4-14 of lactation) rises to an average of 0.22 g/liter, and then to 0.26 g/liter in mature milk (days 15+). This increase in glucose occurs in parallel with the increase in lactose during milk maturation (lactogenesis) [2, 3], perhaps reflecting increased glucose transport across the basolateral membrane of mammary alveolar cells [4]. Once lactation is established, glucose concentrations are no longer linked to lactose concentrations, suggesting that glucose concentrations do not limit lactose synthesis [5]. Glucose transport from the circulation is independent of insulin levels [5]. Milk glucose levels fall during weaning and mastitis, probably because both induce opening of tight junctions allowing increased glucose loss from milk through paracellular transport [6].
The concentrations of other monosaccharides in milk are likewise quite low, and vary widely among individuals. Free (unbound) galactose levels may approximate the levels of glucose; L-fucose and sialic acid (NANA, Neu5Ac) are reported at concentrations between approximately 1 and 40 mg/liter [7]. The total monosaccharide content in milk is approximately 500-900 mg/liter [8]. It has been noted that the sialic acid found in human milk is predominantly in the N-acetylneuraminic acid (Neu5Ac) form, the type of sialic acid synthesized by humans, while bovine milk contains extensive N-glycolylneuraminic acid (Neu5Gc). A potential negative role of the N-glycolyl form in human nutrition has been suggested. In bovine milk, the major sources of bound Neu5Gc are oligosaccharides, glycolipids, and glycans of high molecular weight milk components.
Dynamic changes of human milk monosaccharide concentrations. The source of the monosaccharides in milk could result from accumulation of precursors of the synthesis of complex glycans, breakdown of glycans by endogenous glycosidases, or both. To investigate these possibilities, glycosidase activity was measured in human milk. The activities of α-L-fucosidase, α-D-galactosidase, β-D-galactosidase, hexosaminidase, glucuronidase, neuraminidase, and β-D-glucosidase were measured. These enzyme activities are all present in milk at 37°C, with high individual variability among individual donors. Fucosidase and hexosaminidase activities are prominent. Appreciable amounts of fucose and other sugars were released in milk after incubation at 37°C for 16 h, demonstrating the endogenous presence of enzymes, but not their clinical relevance. When human milk samples were analyzed immediately after expression or after storage at 4 or –20°C, only small amounts of free sugars were released [7, 9]. The study concludes that glycoconjugate degradation during the typical residence time of milk in the breast is modest, and that there is little additional breakdown under typical storage conditions.
LACTOSE
The typical milk sugar is lactose, a disaccharide of glucose and galactose. It is formally named lactobiose, whose chemical structure is 4-O-β-D-galactopyranosyl-D-glucopyranose (Fig. 1).
Fig. 1. Chemical structure of lactose.
The amount of lactose in milk is distinct for different species, and in some species lactose is known to change over the course of lactation [10]. Lactose is the predominant carbohydrate in human milk. When ingested, lactose is digested into its monosaccharides, glucose and galactose, by lactase (β-galactosidase), an enzyme that is found in intestinal mucosa of all normal infants and many adults. Glucose is the major source of energy in most cells, and galactose can be converted to glucose in the liver. We hypothesize that galactose from human milk lactose might also be used directly by the infant to make galactose-containing molecules essential for brain development. Galactosylceramide (cerebroside) and its sulfated analog, sulfatide, are predominant molecules in myelin, and the peak of myelination coincides with the nursing period of the breastfed infant [10].
Lactose was the first of the milk components to be isolated, and was described in 1633 [11]. Carl Scheele defined it as a sugar in 1780 [12]; by the 1880s human milk lactose was being quantified by direct chemical methods in multiple human milk samples [13]. Human milk was thought to have constant and consistent levels of lactose (68 g/liter) once the milk matured (~4 weeks), but some reports indicate variation within individuals, across lactation [8], among individuals, and also among ethnic groups. The abrupt and extensive hormonal changes in mothers during parturition induce milk synthesis in their mammary glands. The production for the first several days after parturition is colostrum, a low-volume fluid that is high in secretory antibodies and oligosaccharides and low in casein and lactose. As the volume increases, the mounting levels of casein and lactose define the fluid as milk [14]. This process of lactogenesis is of high clinical and basic research interest, and can be better defined through reliable measurement of low concentrations of lactose in small volumes of multiple samples of milk.
Some of the reported variation is also due to analytic error. Human milk contains many other carbohydrates that can interfere with the most common types of lactose analysis. Milk oligosaccharides contain lactose at their reducing end, and for many types of analysis the oligosaccharides will elevate apparent lactose values. Milk also contains monosaccharides, discussed above, and glycoproteins, glycolipids, glycopeptides, mucins, and glycosaminoglycans, all of which are potential confounders of lactose measurement for many common analytical techniques. Milk sampling is another source of potential error, where the difference in fat concentration between foremilk and hindmilk, and different sampling techniques, can secondarily lead to variation in their lactose content [15]. To be considered as representative of an individual’s milk, it is essential that the entire milk of one full breast be sampled. The data supporting the conclusion that the concentration of lactose in milk varies across individuals and over the course of lactation has been questioned: The argument pro is that the concentrations of most milk constituents are variable, and that lactose should also be expected to vary, at least within a range of values. The argument con is that lactose is an osmoregulator that defines how much milk is made, suggesting that lactose concentrations should always be similar. Thus, the variation, and even the precise concentration, of lactose in milk are still not certain. To define the variation in milk lactose requires a method of high precision and accuracy; the error introduced by the method must be much less than the biologic variation intrinsic to meticulously collected representative human milk samples.
Lactose levels in human milk are clinically relevant when feeding premature infants; it is important to maximize the amount of calories fed, but not to overfeed the infant. Overfeeding can result in intestinal overgrowth, inflammation and, sometimes, necrotizing enterocolitis [16]. Thus, for many very low and extremely low birth weight infants, the amount of fat, protein, and lactose provided through human milk is carefully monitored [17].
Methods of lactose measurement. Crystallization was used for the earliest measurement of human milk lactose, but this is not inherently quantitative. Another classic method is subtractive gravimetric analysis: from the dry weight of the milk is subtracted the deduced weight of protein, the gross weight of the lipids, and the weight of the ash; the residual weight is reported as lactose. However, the high amount of glycosylation in milk (~20 g/liter) can add excessive error to its lactose content (68 g/liter on average). Direct chemical reaction with the reducing end of sugars to produce specific chromophores measured by spectrophotometry added specificity, but reducing ends of other carbohydrates can produce spurious chromophores. Measuring free monosaccharide before and after adding β-galactosidase (lactase) to milk, specifically cleaving lactose into free glucose and galactose, adds the specificity of enzymatic reactions, but has the combined error of two measures. Chromatographic separation of the lactose followed by various types of detection is intrinsically the most accurate analytical strategy. Using HPLC, Butte and Calloway [18] found lactose levels that were slightly below those indicated by other procedures. A popular HPLC procedure [8] allows simultaneous measurement of monosaccharides, lactose, and total oligosaccharides in samples of human milk and colostrum.
Table 1. Reported values of lactose in human
milk are around 68 g/liter in mature milk, and are lower in early
milk and milk from mothers of premature infants. The degree of
interindividual and intraindividual variation of lactose in human milk
is not yet fully defined
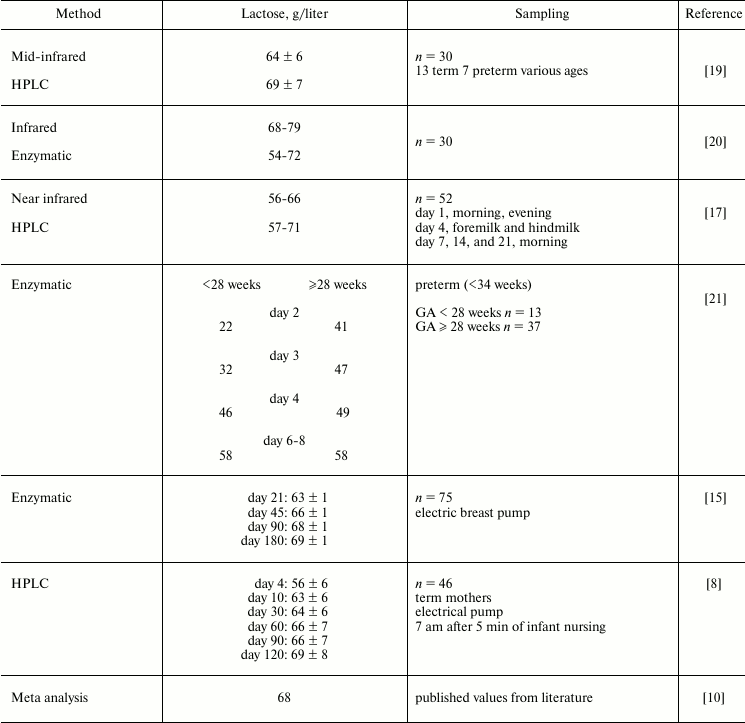
Note: GA, gestational age.
The compilation of reported literature values in Table 1 demonstrates that the concentration of lactose in human milk is still uncertain. High performance liquid chromatography (LC) is a powerful technique for separating carbohydrates of milk, and mass spectrometry (MS) is the most sensitive and specific method for the detection of individual molecules in a mixture. Therefore, we devised and validated an LC-MS technique with a porous graphitic stationary phase and acetic acid (0.002%) and acetonitrile (5%) in water as the mobile phase [22]. It was sensitive to 0.2 pmol/2 µl, had a limit of quantitation of 0.039 µg/ml, and a linear dynamic range of 0.49-31 µg/ml, with linearity (R2) of 0.997. The average coefficient of variation (precision) is 2-3%, and the accuracy (recovery of standard) is 95-99% [10]. The analysis is not influenced by the matrix of human milk. When a large collection of carefully defined milk samples are analyzed, this technique should allow a fuller definition of the human milk lactose concentrations and the factors that control any variation.
OLIGOSACCHARIDES
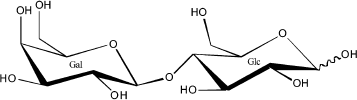
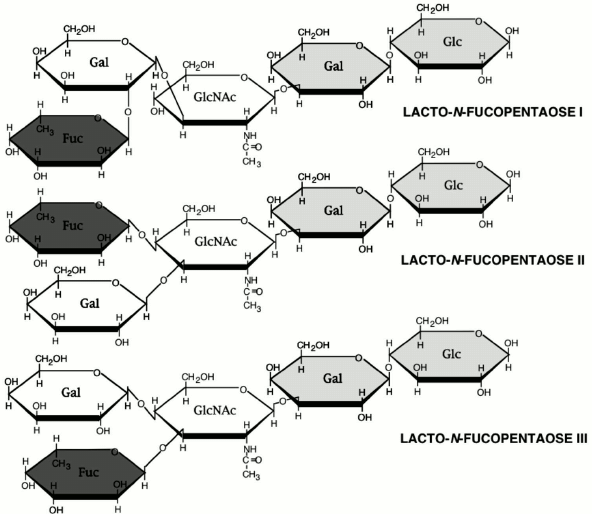
The oligosaccharide fraction is the third largest solid component in milk and the second most abundant glycan in human milk after lactose. Oligosaccharides are essentially indigestible by the infant gut mucosa, and, therefore, their component sugars are not available for use as a macronutrient. Because oligosaccharides are approximately 1% of human milk [10], they represent on the order of 10% of the caloric content of human milk. Such use of precious caloric resources by the mother for synthesis of a glycan milk component that is not directly benefiting the nutritional status of the infant seems counterintuitive. However, it makes biological sense if the glycan serves important non-nutritional functions, such as protection of the infant against disease. The human milk oligosaccharides (HMOS), as shown in the examples in Fig. 2, have lactose on the reducing end, have a core structure of polylactosamine (galactose-N-acetylglucosamine), and usually contain fucose and some sialic acid at the non-reducing terminus [10].
Fig. 2. Three typical oligosaccharides of human milk.
Many of the neutral HMOS also have sialylated homologs, and the sialic acid is predominantly Neu5Ac. Bovine milk has an order of magnitude lower concentration of oligosaccharides; they are mostly found in colostrum, and the oligosaccharides are mainly sialylated. But in contrast to human milk, the sialic acid in bovine milk oligosaccharides contains appreciable Neu5Gc in addition to the N-acetyl form. Many have questioned the wisdom of feeding this N-glycolyl sialic acid, which is naturally found in most infant formulas, as a major ingredient of an infant human diet at a time of maximum brain development.
The HMOS, like all other complex glycans, have a multiplicity of possible linkages between its several component sugars, with a vast number of permutations of possible structures and structural isomers. The neutral human milk oligosaccharide fraction, when analyzed by mass spectrometry, displayed molecular weights (m/z ratios) that were interpreted as representing structures of up to 32 sugars containing up to 15 fucose moieties per molecule [23]. The structural isomerism seen in the known HMOS indicated that each molecular ion was highly likely to contain a multiplicity of isomeric structures, and therefore these mass spectra represented many thousands of structures. The terminal moieties of the known neutral HMOS are homologs of the Lewis blood group structures, fucosylated glycan moieties with high biological activity in many important systems related to development and immune function [24]. Likewise, the acidic human milk oligosaccharide fraction contains many sialylated analogs of the Lewis moieties, and the sialylated Lewis structures have very high biological activity in many biological systems, notably in immune signaling. The vast number of potential structures in the human milk oligosaccharide fraction suggest that it contains structures with homology to cell surface glycans; their high concentration in human milk suggest that they might inhibit binding of pathogens to their mucosal receptors, thereby protecting the infant from disease. This hypothesis was tested in models of infection by human pathogens [1].
Inhibition of pathogens. The intestinal mucosa is among the most heavily glycosylated tissues in the body. Mucosal epithelial cells are covered with glycoproteins, glycolipids, mucins, glycosaminoglycans, and other glycans, and these have multiple biological functions [25]. Many enteric pathogens use specific cell surface glycan moieties of the intestinal epithelial cells as their target for binding, the first obligatory step in their pathogenesis. The ability of a host cell to express specific glycan epitopes defines the organ and species specificity of each pathogen. When this epitope is part of a soluble glycan, it could competitively inhibit the ability of a pathogen to dock to its receptor, and thereby spare the target cell from infection. The human milk oligosaccharide fraction does contain such epitopes that protect the breastfed infant against enteric pathogens [26]; a few representative examples follow.
Enterotoxigenic Escherichia coli is a major cause of diarrhea globally. Its pathogenesis is associated with two toxins, labile toxin and stable toxin. Labile toxin is homologous with cholera toxin; stable toxin is an 18 amino acid peptide containing three disulfide bonds, making the molecule stable to heat and organic solvents. Stable toxin inhibits chloride transport from the gut to the intestinal epithelial cell, thereby inhibiting electrolyte and water resorption from the gut, resulting in secretory diarrhea in susceptible humans, including travelers and infants. An animal model for stable toxin pathology is the suckling mouse, which is sensitive to this toxin between 2 and 4 days of life. Mice fed the toxin develop secretory diarrhea, which is lethal within a few hours. Mice fed stable toxin along with human milk have a significantly lower rate of death. At its concentration in milk, the neutral oligosaccharide fraction of human milk prevented stable toxin-induced death in the mice [27]. The active oligosaccharide purified from the native oligosaccharide mixture was a large oligosaccharide containing α-1,2-linked fucose. It was active at its original concentration found in milk, at approximately 30 parts per billion.
Campylobacter pylori infection is the major cause of bacterial diarrhea in infants who live in developing countries. Breastfed infants have a lower rate of diarrhea. Campylobacter binds to the H-2 epitope (Fucα1,2Galβ1,4GlcNAc) expressed on the surface of human intestinal epithelium [28]. This binding is inhibited by 2′-fucosyllactose (2′-FL), the major human milk oligosaccharide containing the H-2 moiety. Mice transfected with the human fucosyltransferase I gene controlled by the whey acidic protein promoter produced high H-2 epitope in their milk. Campylobacter infected mice nursing wild-type dams remained infected throughout the 15 days of the experiment, while those nursing the transfected dams cleared the infection. Thus, the milk containing H-2 inhibits campylobacter from binding its intestinal glycans, protecting pups from campylobacter infection.
This inhibition of pathogen binding to intestinal receptors by Fucα1,2 oligosaccharides suggests that human milk glycans could be an important component of an innate immune system of human milk [29]. Individual variation in expression of oligosaccharides containing α-1,2-linked fucose in milk allows the relationship between its presence in milk and disease outcome in infants to be tested in a cohort of breastfeeding dyads [30]. Breastfeeding infants who were positive for E. coli in their stools and who exhibited symptoms of diarrhea were consuming milk with significantly lower concentrations of fucosylated oligosaccharides than infants who were colonized but did not exhibit symptoms of diarrhea. This is consistent with the fucosylated oligosaccharides of the milk protecting infected infants from the effects of stable toxin of E. coli.
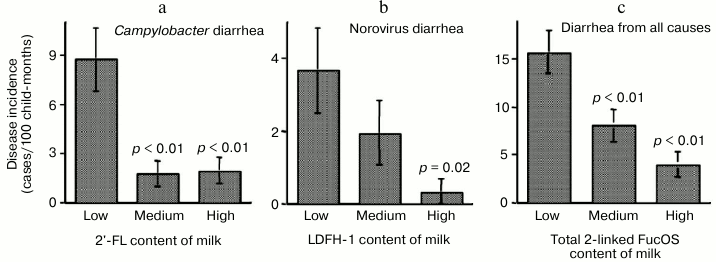
The risk of campylobacter diarrhea in breastfed infants was inversely related to the levels of 2′-FL in milk samples, indicating that 2′-FL in milk protects against campylobacter (Fig. 3).
Fig. 3. Milk oligosaccharides consumption abates infant diarrhea. Infant risk of diarrhea is inversely related to the consumption of human milk oligosaccharides.
Lactodifucohexaose-1 (LDFH-I) is a milk oligosaccharide that contains a Lewis b moiety and binds to norovirus in vitro. The level of LDFH-I in milk is inversely related to the risk of norovirus-associated diarrhea in breastfed infants, indicating that LDFH-I in milk protects breastfed infants from norovirus-induced diarrhea. Thus, specific glycans in human milk can protect against specific pathogens.
The composite total concentration of all α-1,2-linked fucosyloligosaccharides in milk samples was inversely related to risk of diarrhea of all causes in breastfed infants. These data strongly support the conclusion that the human milk α-1,2-linked fucosyloligosaccharides, by binding to specific pathogens, prevent the pathogens from binding to their host cell receptors in the intestinal mucosa, thereby protecting the infant from developing moderate-to-severe diarrhea.
Sialyl glycans and rotavirus. Protection of nursing infants by other types of glycans in milk is exemplified by the glycoprotein lactadherin. This 46-kDa glycoprotein is associated with mucin on the milk fat globule membrane, contains sialic acid, and binds to rotavirus. Rotavirus is the single largest cause of diarrhea in humans, and a major cause of diarrhea in infants and farm animals. Lactadherin inhibits rotavirus infection of MA104 cells in vitro. This dose-dependent inhibition is lost when the sialic acid is removed from the molecule, indicating that the sialic acid of its glycans is essential for inhibition [31]. Rotavirus infected breastfed infants with overt symptoms of diarrhea were consuming milk that was significantly lower in lactadherin concentrations than those who were asymptomatic [32]. Thus, this acidic glycoprotein of human milk protects infants against rotavirus-induced diarrhea, suggesting the possibility that many types of human milk glycans have the potential to protect against specific enteric pathogens.
Prebiotic effects. In addition to the anti-adhesive mechanism of protection by HMOS described above [29], another more general mechanism could involve the ability of these indigestible glycans to promote colonization of the gut. The vacant infant gut undergoes colonization by a succession of microbes after birth, resulting in the complex stable microbiota of the more mature child [33]. Human milk had been proposed to contain glycans that stimulate growth by mutualist bacteria [34]. Such activity is now defined as prebiotic. A prebiotic is an indigestible dietary glycan that stimulates colonization by beneficial bacteria and provides a health benefit. Typical prebiotics in use today, such as fructosyl oligosaccharides (FOS), enhance growth of bifidobacteria and lactobacilli; bacteria digest the prebiotic to release sugars that can then be fermented to produce organic acids, lowering the pH [35]. Prebiotics are reported to suppress numbers of potentially harmful bacteria in the microbiota, and confer other health benefits to the host. Thus, if HMOS are prebiotic, this would also contribute toward the lower risk of morbidity and mortality in breastfed infants [36, 37]. Human milk glycans are essentially indigestible by mammalian gut, and therefore pass into the distal gut where they influence the composition of the intestinal microbiota [38]. We hypothesize that fucosylated oligosaccharides of milk would work in concert with fucosylated glycoconjugates expressed on the surface of the intestinal mucosa toward establishing a normal, beneficial gut microbiota. Consistent with this hypothesis, low expression of fucosylated glycans in the intestine of premature infants is associated with especially high risk of negative outcomes [39]. Incongruent early microbiota are associated with risk of necrotizing enterocolitis [40]. Thus, if fucosylated glycans of human milk function as prebiotics to promote colonization by fucose-utilizing mutualist symbionts of the immature intestinal mucosa of infants, they could confer health benefits to the neonate.
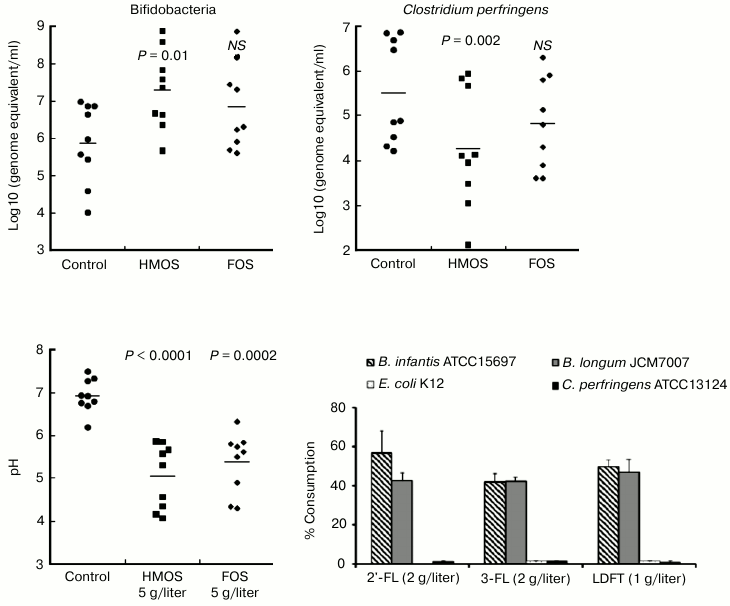
This hypothesis was directly tested in anaerobic culture of human fecal microbiota. First, a mixture of HMOS from pooled milk, which contained fucosylated oligosaccharides, was added to the microbiota culture. The growth of Bifidobacterium spp. was stimulated, accompanied by a decrease in pH level, and increased lactate concentration consistent with a prebiotic effect (Fig. 4) [41].
Fig. 4. Prebiotic effect of human milk oligosaccharides. Human milk oligosaccharides stimulate growth of bifidobacteria, decrease the pH, and decrease the survival of Clostridium perfringens. Only the bifidobacteria consume human milk oligosaccharides.
Moreover, the principal prebiotic activities, stimulation of bifidobacteria growth and production of organic acids, are stronger with HMOS supplementation than by an equivalent amount of FOS, the most established and efficacious prebiotic. Therefore, HMOS are powerfully prebiotic for the infant microbiome. During anaerobic fermentation of HMOS, the fecal microbiota consumed more than 90% of 2′-FL and lactodifucotetraose (LDFT). This leads to the question of the mechanism of this prebiotic effect with regard to which classes of microbes consume and respond to which individual milk oligosaccharides. Cultures of the isolated mutualists Bifidobacterium infantis and Bifidobacterium longum were compared with cultures of the non-mutualists E. coli K12 and Clostridium perfringens.
Fermentation products of human milk oligosaccharides. When supplemented with HMOS, B. infantis and B. longum produced copious lactate and short chain fatty acids, accompanied by a significant pH reduction in the culture medium. When supplemented with only 2′-FL, B. infantis and B. longum also produced ample lactate and short chain fatty acids, with B. infantis producing more lactate and less short chain fatty acids than B. longum. Bifidobacterium infantis and B. longum also metabolized 3-fucosyllactose (3-FL) and LDFT into lactate and short chain fatty acids, with B. longum displaying greater ability to transform 3-FL into lactate and short chain fatty acids than B. infantis. Thus, even in closely related bifidobacteria, fermentation of the same oligosaccharides can result in differences in the relative amounts of fermentation products. The pH reduction induced by the total HMOS at 5 g/liter was greater than that of 2 g/liter supplementation by individual 2′-FL or 3-FL, or by 1 g/liter of LDFT, their approximate physiologic levels in typical human milk. Therefore, no single oligosaccharide accounts for all of the prebiotic activity of the natural HMOS mixture. The pH reduction by the total HMOS at 5 g/liter also exceeded that of FOS at 5 g/liter in the Bifidobacterium spp.
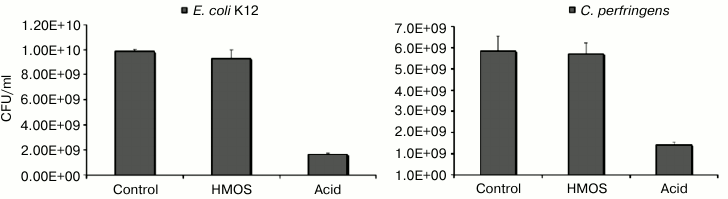
In stark contrast, very little of these individual fucosylated oligosaccharides was converted by E. coli K12 into lactate or short chain fatty acids, and C. perfringens produced significantly less of these metabolic products than either of the bifidobacteria. Likewise, there was no direct inhibition of these non-mutualists by the HMOS. In contradistinction, supplementation of these individual cultures with the acids at the concentrations produced by the HMOS-supplemented bifidobacteria cultures significantly inhibited the growth of E. coli K12 and C. perfringens (Fig. 5). Inhibition of non-mutualist bacteria by oligosaccharide fermentation products demonstrate and confirm that bifidobacteria ferment milk oligosaccharides into acids more efficiently than representatives of other bacterial genera; in mixed microbiota cultures, the inhibition of E. coli and C. perfringens growth by human milk oligosaccharide supplementation seems to be mediated not by the HMOS per se, but by their organic acid fermentation products.
Fig. 5. Escherichia coli and Clostridium perfringens are inhibited by human milk oligosaccharide fermentation products. Escherichia coli and Clostridium perfringens in isolation are not inhibited by human milk oligosaccharides per se, but rather by human milk oligosaccharide fermentation products.
HMOS support cooperative mutualism. In a fecal microbiota community, cooperation between different species allows the community to more efficiently utilize substrates than is possible by a single bacterium [42]. Different species within a human gut microbiome each have overlapping but unique specificities with regard to their ability to metabolize the individual milk oligosaccharides from the mixture found in human milk. Whole infant microbiota metabolize HMOS more efficiently than their isolated bacterial components, providing a concrete example of cooperative communal behavior enhancing metabolism. In addition to being the source of these fermentation products, human milk oligosaccharides concurrently select desired bacteria as early colonizers, stimulating development of the complex normal community of interdependent microbiota important to human health and development. Future studies may define putative contributions of HMOS in promoting colonization by pioneering species, microbes essential to succession, and keystone species of the mature microbiome. These functions could also help with subsequent preservation and renewal of the human gut microbiota. Failure of these functions may underlie chronic diseases of microbial dysbiosis. This strong prebiotic effect of specific HMOS adds to the known human milk functions in protecting the infant from microbial challenges and supporting proper colonization of the gut during development. These data support promotion of breastfeeding, and provision of HMOS upon weaning, with the expected benefits being both immediate and lifelong.
GLYCOPROTEINS
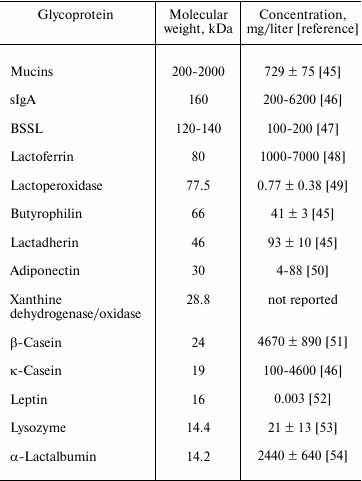
Human milk glycoproteins are principal components of human milk, and these glycoproteins vary in size, structure, and abundance. Major human milk glycoproteins include mucins, secretory immunoglobulin A (sIgA), bile salt stimulated lipase (BSSL), lactoferrin, lactoperoxidase, butyrophilin, lactadherin, xanthine dehydrogenase/oxidase, casein, leptin, adiponectin, and α-lactalbumin (Table 2). Major human milk glycoproteins protect against microbial infection [43] and excessive inflammatory responses in vitro [44]. This suggests that human milk glycoproteins are important constituents in human milk whereby the mother protects her immature infant against pathogenic infection.
Table 2. Molecular weight and concentration
of major human milk glycoproteins
MUCINS
Mucins are high molecular weight glycoproteins whose size ranges from about 200 to 2000 kDa. Mucins are major components of the extracellular matrix, and are involved in diverse functions, including shielding the epithelium against pathogenic infection, regulating cellular signaling, and transcription [55]. The mucin family of large, heavily glycosylated proteins are characterized by a variable number of tandem repeats, the mucin domain, which comprises much of the protein component of mucus. At least 16 mucins have been identified in humans, and the expression profile of the mucins varies among tissues, with the gastrointestinal tract showing the highest and most diverse expression. The mucin family can be divided into three subfamilies according to their location relative to the cell surface: (a) gel-forming (secreted) mucins, such as mucin 1, mucin 4, and mucin 16; (b) cell surface (transmembrane, membrane-tethered) mucins, such as mucin 2, mucin 5, and mucin 6; and (c) secreted non gel-forming mucins, such as mucin 7 [56]. The large size and hydrophobicity of the mucins make them challenging to isolate and purify from the complex matrix of milk. The major human milk mucins are mucin 1 and a higher molecular weight electrophoresis band [57, 58], designated mucin X [59, 60]; mucin 4 seems to be the band previously designated mucin X [61]. Other types of mucins have not been isolated from human milk to date, but could be present in minor amounts.
Mucin 1 and mucin 4 are dimers; each dimer is formed by cleavage of an intact single peptide product of a single gene. The larger subunit is wholly extracellular, heavily glycosylated, and almost entirely composed of a variable number of tandem repeats [55]. Mucin 1 and mucin 4 can interact with microorganisms. The most commonly studied mechanism is a sialic acid moiety of mucin 1 interacting with the pathogen, thereby inhibiting the ability of the pathogen to bind to its infant host cell surface glycan receptor. Thus, mucin 1 plays a role in innate immune defense of the infant against invading microorganisms. However, other human milk mucins have only begun to be investigated for their role in interaction with microorganisms. These data would help understand the full biological role of human milk mucins in protecting infants.
GLYCOSAMINOGLYCANS
Glycosaminoglycans (GAGs) are a heterogeneous class of anionic polysaccharides, which are essential constituents of the extracellular matrix and ubiquitous on the surface of mammalian cells. GAGs are traditionally known for their function [62] as lubricants in joints. On the cell surface glycocalyx, a number of signaling and regulatory functions have emerged related to interaction with cytokines, chemokines, and growth factors [63, 64]. The central structure of GAGs is disaccharide repeating units of hexosamine and uronic acid [65]. GAGs are classified into four distinct categories based on their chemical components: hyaluronic acid; galactosaminoglycans (chondroitin and dermatan sulfate); the glucosaminoglycans (heparin and heparan sulfate), and keratan sulfate. GAGs are variously decorated with sulfate groups, either as O-substituted or, in heparin and heparan sulfate, N-substituted, with the exception of hyaluronic acid, which is not sulfated, but contains glucuronic acid. Hyaluronic acid is the only GAG occurring as a free polysaccharide; the others are covalently attached to a protein core to form proteoglycans. The distribution, average molecular weight, and sulfation pattern of GAGs is tissue specific [65].
The presence of several GAGs in human milk was reported in 1995 [66]. More recently, a combination of enzymatic assays and analytical techniques were used to compare human milk GAGs [67] with that of bovine milk. Human milk contained 7-fold higher GAG content than bovine milk. The main constituent of the human milk GAGs was an undersulfated chondroitin sulfate (55% of total GAGs), followed by heparan sulfate (~40%) and minor amounts of hyaluronic acid and dermatan sulfate. Conversely, dermatan sulfate represented almost 40% of the bovine milk GAGs, whereas heparin and chondroitin sulfate were present at ~30 and ~20%, respectively. Preterm human milk contains roughly three times more GAGs than term milk, but the GAG composition was similar in both [68]. On day 4 of lactation, the concentration of GAGs is 9.3 and 3.8 g/liter for preterm and term milk, respectively. On day 30, the concentration is 4.3 and 0.4 g/liter, respectively.
A GAG-containing fraction isolated from human milk inhibits the binding of gp120, a key protein for HIV infection, to its CD4 receptor [66]. This bioactivity was lost after chondroitinase ABC digestion, suggesting that a chondroitin sulfate containing molecule in human milk blocks HIV infection. Like HMOS, the human milk GAGs are indigestible, contain a large number and concentration of glycans, and have been suggested as potential inhibitors of virus and pathogenic bacteria adhesion to the intestinal epithelium. GAGs regulate and activate a number of growth factors and could help regulate gut epithelial development.
GLYCOLIPIDS
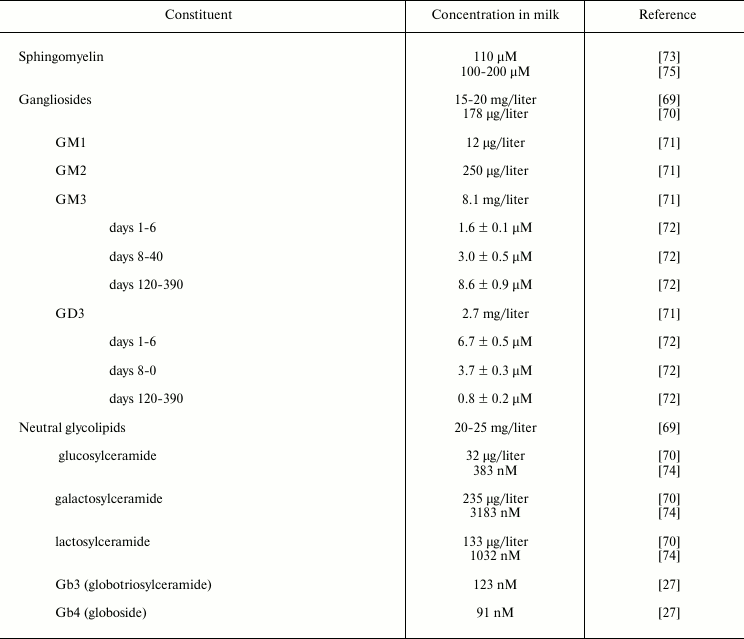
Sphingolipids are lipids based on a long-chain nitrogenous base complexed with a long-chain fatty acid through an amide linkage. The glycolipids found in human milk are primarily glycosphingolipids, which are most prevalent in the membranous structures of milk, and especially in the human milk fat globule membrane (Table 3).
Gangliosides are glycosphingolipids that contain sialic acid (N-acetyl neuraminic acid, NANA) as part of their carbohydrate moiety, thus conferring the molecule with a net negative charge at physiologic pH. Gangliosides are often found in the outer leaflet of the plasma membrane. The ganglioside GM1 is known to bind to cholera toxin, labile toxin of E. coli and a similar toxin from Campylobacter jejuni; thus its presence in human milk is postulated to have a significant role in protection of the infant.
A crude ganglioside fraction was isolated from human milk by Grimmonprez and Montreuil [69]. Bouhours and Bouhours [70] isolated a ganglioside fraction from isolated milk fat globule membrane. Lægreid et al. [71] extracted gangliosides from the creams of ten mothers who were in the second to tenth month of lactation, and quantified them by high performance thin layer chromatography (HPTLC), densitometry, and HPTLC immuno-densitometry for GM1. These values agree with those of Takamizawa et al. [72]. Measuring gangliosides from day 2 to day 390 of lactation revealed a reciprocal relationship between milk GD3, the predominant ganglioside early in lactation, and milk GM3, which predominates late in lactation.
Grimmonprez and Montreuil [69] also found neutral glycolipids from the lower (organic) phase of a Folch distribution (8 : 4 : 3, chloroform–methanol–water) of human milk. Bouhours and Bouhours [70, 73] measured the neutral glycolipid levels through classical gravimetric, TLC, and GC techniques from human milk fat globule membrane. Whole milk samples obtained from women representing various stages of lactation, parity, etc. were subjected to Folch distribution, and the lower phase neutral glycolipids were perbenzoylated, separated by gradient HPLC, and quantified by UV absorbance by Newburg and Chaturvedi [74]. In addition to the expected cerebrosides and lactosylceramide, low levels of Gb3 and Gb4 were found consistently in human milk. Newburg et al. [27] demonstrated that these globo-series glycolipids from human milk bind to Shiga toxin at levels that could be relevant to the protection of infants by human milk. Gb3 also binds to Shiga-like toxin produced by some enterohemorrhagic E. coli; Gb4 binds to a variant of Shiga-like toxin. These glycolipids are present in small amounts but bind strongly to these bacterial toxins; their clinical relevance toward protection of pediatric patients has not been tested.
Table 3. Sphingolipids in human milk
ATTENUATION OF INFLAMMATION
When considering the hypothesis that human milk is an innate immune system whereby the milk of the mother protects the infant from harm, one interesting facet of protection is the sparse but tantalizing data suggesting that human milk is strongly anti-inflammatory [76]. Major anti-inflammatory agents that have been identified in human milk include enzymes that degrade mediators of inflammation, anti-proteases, lysozyme, lactoferrin, secretory IgA, and a number of antioxidants including cysteine, ascorbate, α-tocopherol, and β-carotene [77]. Immature intestinal mucosa exhibits an excessive inflammatory response that might be dampened by human milk components such as transforming growth factor (TGF)-β, interleukin (IL)-10, erythropoietin, and lactoferrin [78]. These could act individually or in concert to control the neonatal immature anti-inflammatory response. This implies that artificial feeding could deprive the immature infant of valuable protection against common enteric–respiratory disorders and their inflammatory consequences.
The HMOS may also directly attenuate some forms of inflammation. HMOS reduce formation of the platelet–neutrophil complex, decreasing neutrophil β-2 integrin expression [79]. An acidic HMOS fraction reduced platelet–neutrophil complex formation up to 20%, as does sialyl-Lewis x, and decreases its β2 integrin expression in a dose-dependent manner. In this system, the neutral HMOS fraction is not active. The acidic anti-inflammatory HMOS may contribute to the lower incidence of inflammatory diseases such as necrotizing enterocolitis in breast-fed versus formula-fed infants [80].
Excessive leukocyte infiltration causes severe tissue damage in a variety of inflammatory diseases, and a critical step in infiltration is monocyte, lymphocyte, and neutrophil adhesion to endothelial cells. This initial step in leukocyte extravasation is mediated by selectin binding to the oligosaccharides of their glycoconjugate ligands. HMOS prevented leukocyte rolling and adhesion to endothelial cells under hemodynamic conditions as monitored by video microscopy. Monocytes, lymphocytes, or neutrophils isolated from human peripheral blood were passed over TNF-α-activated HUVEC under shear stress. Within a physiological range of concentration, the acidic HMOS fraction significantly inhibited leukocyte rolling and adhesion on endothelium. Thus, anti-inflammatory acidic oligosaccharides of human milk may contribute to the lower incidence of inflammatory diseases in human milk-fed infants [81].
An excessive maternal inflammatory response may contribute to the pathogenesis of preeclampsia [82]; this imbalance may also have an impact upon inflammatory mediators in the subsequent human milk. The milk of formerly preeclamptic and healthy women during pregnancy had differences in cytokine levels. In the healthy control group cytokine levels decreased in mature milk versus colostrum but did not decrease in the formerly preeclamptic group. Thus, the IL-8 and TNF-α levels in mature milk were higher in the preeclampsia group, consistent with a chronic heightened inflammatory state in these mothers. Looked at in a positive light, this extra cytokine in the milk could offer an immunological signal to heighten host defense in high-risk neonates [82].
Mastitis is a common complication of human lactation, and the milk produced during mastitis has differences in some of its characteristics. In general, mastitis milk has the same anti-inflammatory components and characteristics as normal milk, with some extra pro-inflammatory cytokines (IL-6, IL-1β) and soluble receptors (sIL1R-II, sTNFRI, s-IL6R) shed from polymorphonuclear cells; these may help protect the alimentary canal of the nursing infant from any residual bacteria in the mastitis milk [77].
Whole human milk strongly suppresses many inflammatory processes. Only a few of its anti-inflammatory components have been identified. It seems likely that the human milk oligosaccharides and glycans will prove to account for much of the strong association between breastfeeding and lower risk of inflammatory condition in infants.
SUMMARY AND CONCLUSIONS
The lower incidence of disease in infants while they are breastfeeding has been attributed to many causes, including less exposure to contaminated water, better nutritional status, and psychological factors [83]. This review discusses molecules in human milk whose bioactivity per se directly reduces disease among breastfed infants [83] with an emphasis on the high numbers and amounts of the carbohydrates of human milk [84, 85].
Early research found that breastfed infants had high levels of bifidobacteria in their gut, and a prolonged search for a factor in human milk that was responsible for this stimulation (the elusive bifidus factor) eventually led to a glycan. Expanded research on human milk complex carbohydrates has since found that many human milk glycans have activities that span many areas of immune protection. Finding multiple bioactive human milk glycans is the foundation for my hypothesis that the human milk glycans collectively constitute an innate immune system whereby the mother protects her infant from disease. These complex carbohydrate components of human milk confer benefits to breastfed infants through multiple complementary mechanisms. Many glycans have been found that inhibit the ability of a pathogen to bind to their host cell receptors, a critical first step in their pathogenesis. Human milk glycans have been found to suppress excessive inflammation. The glycans of human milk are also strongly prebiotic, directing proper colonization of the gut with the proper specific microbiota selected from the microbial inoculum provided by the mother and other humans close to the infant. These mutualist microbes of infants are able to ferment human milk glycans into small organic acids that function to suppress the growth and colonization of pathogens, participate in development of the gut, suppress inflammation, and promote homeostasis in the intestinal mucosa.
There is increasing recognition that human milk is the ideal food for infants. Prior research had focused on the nutrient composition of milk, and the results of that research led to both increased promotion of breastfeeding and improvements in infant formula. But milk is more than a food. It supports not only growth, but also many additional aspects of development, including specialized aspects of maturation of the nervous system and immunologic systems. It is clear that science cannot replicate many of the features of human milk, not only because of limitations with our current state of the art, but also because of the intrinsic complexity of human milk. This is apparent especially in premature infants, where autoimmune diseases like necrotizing enterocolitis and limitations in neurologic development have been attributed to our inability to provide proper nutritional support. Although feeding human milk to these infants reduces risk for these problems, this illustrates how far we are from our goal of fully understanding the nutritional needs of infants and how to meet these needs. The more we learn about human milk, the more we realize how far we are from understanding its many properties. Current research is focusing more on the role of milk components in protecting the infant from infection and inflammation, and in skewing gut colonization toward a healthy mutualistic microbiota. The biologically active glycans discussed above that influence these processes are undoubtedly only the tip of the iceberg.
The research of our group is supported by the National Institutes of Health, USA: HD013021, HD059140, AI075563, HD061930.
REFERENCES
1.Newburg, D. S., Ruiz-Palacios, G. M., and Morrow,
A. L. (2005) Annu. Rev. Nutr., 25, 37-58.
2.Kulski, J. K., and Hartmann, P. E. (1981) Aust.
J. Exp. Biol. Med. Sci., 59, 101-114.
3.Kulski, J. K., Smith, M., and Hartmann, P. E.
(1981) Aust. J. Exp. Biol. Med. Sci., 59, 405-412.
4.Neville, M. C., Hay, W. W., Jr., and Fennessey, P.
(1990) Protoplasma, 159, 118-128.
5.Arthur, P. G., Kent, J. C., and Hartmann, P. E.
(1991) J. Pediatr. Gastroenterol. Nutr., 13, 260-266.
6.Neubauer, S. H., Ferris, A. M., and Hinckley, L.
(1990) FASEB J., 4, A915.
7.Wiederschain, G. Y., and Newburg, D. S. (2001)
J. Nutr. Biochem., 12, 559-564.
8.Coppa, G. V., Gabrielli, O., Pierani, P., Catassi,
C., Carlucci, A., and Giorgi, P. L. (1993) Pediatrics,
91, 637-641.
9.Wiederschain, G. Y., and Newburg, D. S. (2001)
Adv. Exp. Med. Biol., 501, 573-577.
10.Newburg, D. S., and Neubauer, S. H. (1995) in
Handbook of Milk Composition (Jensen, R. G., ed.) Academic
Press, Orlando, pp. 273-349.
11.Bartoletti, F. (1633) Methodus in Dyspnoeam;
seu, de Respirationibus (Tebaldini, N., ed.) Bononiae
(Bologna).
12.Linko, P. (1982) in Nutritive Sweeteners
(Birch, G. G., and Parker, K. J., eds.) Applied Science Publishers,
London & New Jersey, pp. 109-132.
13.Leeds, A. (1884) J. Am. Chem. Soc.,
6, 252-279.
14.Kent, J. C. (2007) J. Midwifery Womens
Health, 52, 564-570.
15.Allen, J. C., Keller, R. P., and Neville, M. C.
(1991) Am. J. Clin. Nutr., 54, 69-80.
16.Okada, K., Fujii, T., Ohtsuka, Y., Yamakawa, Y.,
Izumi, H., Yamashiro, Y., and Shimizu, T. (2010) Neonatology,
97, 218-224.
17.Sauer, C., and Kim, J. (2011) J.
Perinatol., 31, 339-343.
18.Butte, N. F., and Calloway, D. H. (1981) Am.
J. Clin. Nutr., 34, 2210-2215.
19.Casadio, Y., Williams, T., Lai, C., Olsson, S.,
Hepworth, A., and Hartmann, P. (2010) J. Hum. Lactation,
26, 376-383.
20.Michaelsen, K. F., Pedersen, S. B., Skafte, L.,
Jæger, P., and Peitersen, B. (1988) J. Pediatr. Gastroenterol.
Nutr., 7, 229-235.
21.Henderson, J., Hartmann, P. E., Newnham, J. P.,
and Simmer, K. (2008) Pediatrics, 121, e92-100.
22.Newburg, D. S., Chen, C., and Wiederschain, G.
(2013) in Dietary Sugars: Chemistry, Analysis,
Function, and Effects (Preedy, V., ed.) The Royal Society of
Chemistry, Cambridge, UK, pp. 570-588.
23.Stahl, B., Steup, M., Karas, M., and Hillenkamp,
F. (1991) Anal. Chem., 63, 1463-1466.
24.Stahl, B., Thurl, S., Henker, J., Siegel, M.,
Finke, B., and Sawatzki, G. (2001) Adv. Exp. Med. Biol.,
501, 299-306.
25.Newburg, D. S., and Walker, W. A. (2007)
Pediatr. Res., 61, 1-8.
26.Newburg, D. S. (1999) Curr. Med. Chem.,
6, 117-127.
27.Newburg, D. S., Ashkenazi, S., and Cleary, T. G.
(1992) J. Infect. Dis., 166, 832-836.
28.Ruiz-Palacios, G., Calva, J. J., Pickering, L.
K., Lopez-Vidal, Y., Volkow, P., Pezzarossi, H., and West, M. S. (1990)
J. Pediatr., 116, 707-713.
29.Newburg, D. S., Ruiz-Palacios, G. M., Altaye, M.,
Chaturvedi, P., Meinzen-Derr, J., Guerrero, M. L., and Morrow, A. L.
(2004) Glycobiology, 14, 253-263.
30.Morrow, A. L., Ruiz-Palacios, G. M., Altaye, M.,
Jiang, X., Guerrero, M. L., Meinzen-Derr, J. K., Farkas, T.,
Chaturvedi, P., Pickering, L. K., and Newburg, D. S. (2004) J.
Pediatr., 145, 297-303.
31.Yolken, R. H., Peterson, J. A., Vonderfecht, S.
L., Fouts, E. T., Midthun, K., and Newburg, D. S. (1992) J. Clin.
Invest., 90, 1984-1991.
32.Newburg, D., Peterson, J., Ruiz-Palacios, G.,
Matson, D., Morrow, A., Shults, J., Guerrero, M., Chaturvedi, P.,
Newburg, S., Scallan, C., Taylor, M., Ceriani, R., and Pickering, L.
(1998) Lancet, 351, 1160-1164.
33.Mackie, R. I., Sghir, A., and Gaskins, H. R.
(1999) Am. J. Clin. Nutr., 69, 1035S-1045S.
34.Gyorgy, P., Norris, R. F., and Rose, C. S. (1954)
Arch. Biochem. Biophys., 48, 193-201.
35.Gibson, G. R., and Roberfroid, M. B. (1995) J.
Nutr., 125, 1401-1412.
36.Cunningham, A. (1979) J. Pediatr.,
95, 685-689.
37.Newburg, D. S. (2001) Adv. Exp. Med.
Biol., 501, 3-10.
38.Zivkovic, A. M., German, J. B., Lebrilla, C. B.,
and Mills, D. A. (2011) Proc. Natl. Acad. Sci. USA, 108
(Suppl. 1), 4653-4658.
39.Morrow, A. L., Meinzen-Derr, J., Huang, P.,
Schibler, K. R., Cahill, T., Keddache, M., Kallapur, S. G., Newburg, D.
S., Tabangin, M., Warner, B. B., and Jiang, X. (2011) J.
Pediatr., 158, 745-751.
40.Mshvildadze, M., Neu, J., Shuster, J., Theriaque,
D., Li, N., and Mai, V. (2010) J. Pediatr., 156,
20-25.
41.Coppa, G. V., Bruni, S., Morelli, L., Soldi, S.,
and Gabrielli, O. (2004) J. Clin. Gastroenterol., 38,
S80-83.
42.Faust, K., Sathirapongsasuti, J. F., Izard, J.,
Segata, N., Gevers, D., Raes, J., and Huttenhower, C. (2012) PLoS
Comput. Biol., 8, e1002606.
43.Florisa, R., Recio, I., Berkhout, B., and Visser,
S. (2003) Curr. Pharm. Des., 9, 1257-1275.
44.Lonnerdal, B. (2003) Am. J. Clin. Nutr.,
77, 1537S-1543S.
45.Peterson, J. A., Hamosh, M., Scallan, C. D.,
Ceriani, R. L., Henderson, T. R., Mehta, N. R., Armand, M., and Hamosh,
P. (1998) Pediatr. Res., 44, 499-506.
46.Montagne, P. M., Tregoat, V. S., Cuilliere, M.
L., Bene, M. C., and Faure, G. C. (2000) Clin. Biochem.,
33, 181-186.
47.Stromqvist, M., Lindgren, K., Hansson, L., and
Juneblad, K. (1995) J. Chromatogr. A, 718, 53-58.
48.Masson, P. L., and Heremans, J. F. (1971)
Comp. Biochem. Physiol. B, 39, 119-129.
49.Shin, K., Hayasawa, H., and Lonnerdal, B. (2001)
Am. J. Clin. Nutr., 73, 984-989.
50.Martin, L. J., Woo, J. G., Geraghty, S. R.,
Altaye, M., Davidson, B. S., Banach, W., Dolan, L. M., Ruiz-Palacios,
G. M., and Morrow, A. L. (2006) Am. J. Clin. Nutr., 83,
1106-1111.
51.Chtourou, A., Brignon, G., and Ribadeau-Dumas, B.
(1985) J. Dairy Res., 52, 239-247.
52.Ilcol, Y. O., Hizli, Z. B., and Ozkan, T. (2006)
Int. Breastfeed J., 1, 21.
53.Braun, O. H., and Sandkuhler, H. (1985) J.
Pediatr. Gastroenterol. Nutr., 4, 583-586.
54.Jackson, J. G., Janszen, D. B., Lonnerdal, B.,
Lien, E. L., Pramuk, K. P., and Kuhlman, C. F. (2004) J. Nutr.
Biochem., 15, 517-521.
55.Hattrup, C. L., and Gendler, S. J. (2008)
Annu. Rev. Physiol., 70, 431-457.
56.Linden, S. K., Sutton, P., Karlsson, N. G.,
Korolik, V., and McGuckin, M. A. (2008) Mucos. Immunol.,
1, 183-197.
57.Patton, S., Huston, G. E., Jenness, R., and
Vaucher, Y. (1989) Biochim. Biophys. Acta, 980,
333-338.
58.Shimizu, M. K., Yamauchi, K., Miyauchi, Y.,
Sakurai, T., Tokugawa, K., and McIlhinney, R. A. J. (1986) Biochem.
J., 233, 725-730.
59.Patton, S., Gendler, S. J., and Spicer, A. P.
(1995) Biochim. Biophys. Acta, 1241, 407-423.
60.Zhang, J., Perez, A., Yasin, M., Soto, P., Rong,
M., Theodoropoulos, G., Carothers Carraway, C. A., and Carraway, K. L.
(2005) J. Cell Physiol., 204, 166-177.
61.Liu, B., Yu, Z., Chen, C., Kling, D. E., and
Newburg, D. S. (2012) J. Nutr., 142, 1504-1509.
62.Laurent, T. C., Laurent, U. B., and Fraser, J. R.
(1996) Immunol. Cell Biol., 74, A1-7.
63.Casu, B., and Lindahl, U. (2001) Adv.
Carbohydr. Chem. Biochem., 57, 159-206.
64.Jackson, R. L., Busch, S. J., and Cardon, A. D.
(1991) Physiol. Rev., 71, 481-539.
65.Gandhi, N. S., and Mancera, R. L. (2008) Chem.
Biol. Drug Des., 72, 455-482.
66.Newburg, D. S., Linhardt, R. J., Ampofo, S. A.,
and Yolken, R. H. (1995) J. Nutr., 125, 419-424.
67.Coppa, G. V., Gabrielli, O., Buzzega, D.,
Zampini, L., Galeazzi, T., Maccari, F., Bertino, E., and Volpi, N.
(2011) Glycobiology, 21, 295-303.
68.Coppa, G. V., Gabrielli, O., Zampini, L.,
Galeazzi, T., Maccari, F., Buzzega, D., Galeotti, F., Bertino, E., and
Volpi, N. (2012) Neonatology, 101, 74-76.
69.Grimmonprez, L., and Montreuil, J. (1977)
Biochimie, 59, 899-907.
70.Bouhours, J.-F., and Bouhours, D. (1979)
Biochem. Biophys. Res. Commun., 88, 1217-1222.
71.Laegreid, A., Kolsto Otnaess, A.-B., and
Fuglesang, J. (1986) Pediatr. Res., 20, 416-421.
72.Takamizawa, K., Iwamori, M., Mutai, M., and
Nagai, Y. (1986) Biochim. Biophys. Acta, 879, 73-77.
73.Bouhours, J.-F., and Bouhours, D. (1981)
Lipids, 10, 726-731.
74.Newburg, D. S., and Chaturvedi, P. (1992)
Lipids, 27, 923-927.
75.Zeisel, S. H., Char, D., and Sheard, N. F. (1986)
J. Nutr., 116, 50-58.
76.Goldman, A. S., Goldblum, R. M., and Hanson, L.
A. (1990) Adv. Exp. Med. Biol., 262, 69-76.
77.Buescher, E. S., and Hair, P. S. (2001) Cell
Immunol., 210, 87-95.
78.Walker, W. A. (2010) J. Pediatr.,
156, S3-7.
79.Bode, L., Rudloff, S., Kunz, C., Strobel, S., and
Klein, N. (2004) J. Leukoc. Biol., 76, 820-826.
80.Jantscher-Krenn, E., Zherebtsov, M., Nissan, C.,
Goth, K., Guner, Y. S., Naidu, N., Choudhury, B., Grishin, A. V., Ford,
H. R., and Bode, L. (2012) Gut, 61, 1417-1425.
81.Bode, L., Kunz, C., Muhly-Reinholz, M., Mayer,
K., Seeger, W., and Rudloff, S. (2004) Thromb. Haemost.,
92, 1402-1410.
82.Erbagci, A. B., Cekmen, M. B., Balat, O., Balat,
A., Aksoy, F., and Tarakcioglu, M. (2005) Clin. Biochem.,
38, 712-716.
83.Goldman, A. S., Chheda, S., Garofalo, R., and
Schmalstieg, F. C. (1996) J. Mamm. Gland Biol. Neoplasia,
1, 251-258.
84.Newburg, D. S. (2000) J. Pediatr.
Gastroenterol. Nutr., 30, 131-133.
85.Newburg, D. S., Daniel, P. F., O’Neil, N.
E., and McCluer, R. H. (1986) in Human Lactation 2: Maternal and
Environmental Factors (Hamosh, M., and Goldman, A. S., eds.) Plenum
Press, New York, pp. 581-588.