Mitochondrial Models of Pathologies with Oxidative Stress. Efficiency of Alkalization to Reduce Mitochondrial Damage
N. I. Fedotcheva1,2 and E. N. Mokhova2,3*
1Institute of Theoretical and Experimental Biophysics, Russian Academy of Sciences, ul. Institutskaya 3, 142290 Pushchino, Moscow Region, Russia; fax: (4967) 330-553; E-mail: nfedotcheva@mail.ru2Lomonosov Moscow State University, Research Institute of Mitoengineering, 119991 Moscow, Russia; fax: (495) 939-5945
3Lomonosov Moscow State University, Belozersky Institute of Physico-Chemical Biology, 119991 Moscow, Russia; fax: (495) 939-3181; E-mail: mokhova@genebee.msu.ru
* To whom correspondence should be addressed.
Received July 31, 2013; Revision received August 8, 2013
Previously, we developed a method to monitor the development of oxidative stress in isolated liver mitochondria. The method is based on recording of membrane potential changes in response to sequential introduction of low concentrations (5-20 μM) of tert-butyl hydroperoxide (tBHP). It allows monitoring of the extent of amplification or attenuation of oxidative stress caused by external influences (changes in incubation conditions, additions of biologically active substances). Based on this method, we created a mitochondrial model for the study and improvement of treatment of pathologies associated with oxidative stress. The following two processes were simulated in the experiments: 1) introduction of desferal for treatment of serious diseases caused by cell overload with iron (high desferal concentrations were shown to suppress mitochondrial energetics); 2) efficiency of alkalization to reduce mitochondrial damage induced by oxidative stress. The experiments have shown that even a small increase in pH (alkalization) increases the amount of tBHP that can be added to mitochondria before the MPTP (“mitochondrial permeability transition pore”) is induced. The effect of alkalization was shown to be close to the effect of cyclosporin A in the pH range 7.2-7.8. The mechanism of the similarities of these effects in the organism and in mitochondrial suspensions is explained by the increase in toxic reactive oxygen species in both systems under oxidative stress.
KEY WORDS: oxidative stress, mitochondria, tert-butyl hydroperoxide, cyclosporin A-sensitive pore, desferal, alkalizationDOI: 10.1134/S0006297913110102
Abbreviations: DSF, desferal; MPTP, mitochondrial permeability transition pore; tBHP, tert-butyl hydroperoxide; Δψ, transmembrane difference of electrical potentials on the inner mitochondrial membrane.
Oxidative stress causes molecular and cellular damage leading to diverse
serious diseases, although cells possess various mechanisms for
regulation of mitochondrial redox status [1].
Oxidative stress often results from changes in the external
environment, e.g. oxygen concentration [2].
Oxidative stress-induced cell damage is often caused by the opening of the nonspecific Ca2+-dependent mitochondrial permeability transition pore in the inner mitochondrial membrane (MPTP). It was discovered over 30 years ago by Hunter and Haworth [3]. This significant discovery was appreciated only after MPTP opening had been shown to cause cell death.
Verseci et al. suggested that superoxide radical and hydrogen peroxide, which are known to accumulate in mitochondria, exit from mitochondria into the incubation medium. H2O2 can interact with Fe2+, facilitating the formation of highly toxic hydroxyl radical, which can also have toxic effects from the outer medium [4, 5].
As high catalase activity complicated the experiments with hydrogen peroxide on mitochondria isolated from liver and some other tissues, we started using tert-butyl hydroperoxide (tBHP) instead of hydrogen peroxide ([6, 7] and references therein). tBHP is similar to hydrogen peroxide in many respects, including its effects on mitochondria.
Monitoring of mitochondrial redox status and its changes in the course of medical treatment is often used to identify the risk of the development of diseases and/or efficiency of the treatment of the pathology.
Determination of the ratio of reduced and oxidized glutathione (GSH and GSSG) is often used in disease diagnosis, because GSH is one of the major components of the antioxidant system in animal tissues including those of humans [8]. Such measurements, as well as determination of tBHP toxicity (when its concentration increases in some samples) are very time consuming.
We developed earlier a rapid method for monitoring cellular redox status following the dynamics of mitochondrial membrane potential changes (Δψ) caused by introduction of successive additions of low tBHP concentrations [9]. This approach allows study of induction of oxidative stress in intact mitochondria, which have the highest Δψ. Antioxidant and prooxidant activities of biologically active compounds can be studied by quickly varying different parameters in the course of experiments.
The character of responses to small additions shows that tBHP is transported electrophoretically into mitochondria. Once this transport ended, the Δψ returns to baseline. Such membrane potential dynamics provides a quick answer to the question about the tested compound (or different conditions of mitochondria incubation) enhancing or inhibiting the onset of oxidative stress. Earlier experiments showed this transport to proceed at high speed already with the addition of 2 μM Fe2+ into the incubation medium [9]. Similar transport occurs also without any added iron, i.e. endogenous iron is enough for its manifestation.
We used this method for the development of liver mitochondria-based mitochondrial models of pathologies caused by defects in mitochondrial energetics.
In this work, we have studied the following two processes. The first includes introduction of desferal for the treatment of pathologies resulting from cell overload with iron. Many publications by Verseci et al. are dedicated to the thorough study of the MPTP stimulated by oxidative stress in the presence of iron. In particular, they studied MPTP formation caused by administration of a large excess of iron into isolated liver mitochondria [10]. A series of severe pathologies resulting from iron excess have been described, many of them remaining incurable even today. When the state of the patient deteriorates, different methods of reduction of iron content in blood are used, including blood-letting. An injection of an iron-specific reagent, desferal, is an alternative. Desferal does not enter the bloodstream when administered orally. Details and references can be found in [11]. We have also studied the second mitochondria-level process associated with the method proposed by Edgar Cayce (USA). This is the method of non-pharmaceutical treatment of pathologies caused by excessive pH decrease in body fluids by restoration of acid–base balance in body fluids (blood, lymph, saliva, etc.), shifting it in the “alkaline” direction by selecting special diets for different pathologies [12].
Cayce has published many books on the recommended methods of treatment. Efficiency of many of his recommendations has been already confirmed in medical research.
Our experiments have shown a slight increase in the incubation medium pH to increase dramatically the amount of tBHP that must be added to liver mitochondria to open the MPTP. Thus, we have confirmed efficiency of alkalization on the model of liver mitochondria suspension.
MATERIALS AND METHODS
Male Wistar rats from Institute of Theoretical and Experimental Biophysics vivarium were used in the experiments. All the used reagents were produced by Sigma (USA).
Liver mitochondria were isolated by the differential centrifugation method in medium containing 300 mM sucrose, 10 mM Tris, pH 7.4. Mitochondria were isolated by a two-stage centrifugation without washing; the isolation medium contained no EDTA or EGTA.
The value of Δψ was recorded using a TPP+-selective electrode with subsequent computer processing. Mitochondria were incubated in hypotonic sucrose medium containing 75 mM sucrose, 10 mM Mops, pH 7.4 (pH was adjusted by KOH), 1.5 mM phosphate, 5 mM KCl. In some experiments salt incubation medium containing 120 mM KCl, 10 mM Hepes, pH 7.25, 1.5 mM phosphate, 0.5 mM MgCl2 was used, with TPP+ added to concentration 1 μM. Concentration of mitochondrial protein in the measuring cell was 1 mg/ml in all the samples. Details of the described experimental conditions are provided in the legends to the corresponding figures.
Spectral analysis of the formation of desferal complexes with iron (FeSO4) was performed using an Ocean Optics USB4000 spectrophotometer. Complex formation was accompanied by the appearance of brown color at 435-nm wavelength. The peak amplitude at this wavelength depends on the concentration of each of the complex components [13]. The spectra were recorded in medium containing 75 mM sucrose, 10 mM Mops, pH 7.4.
RESULTS
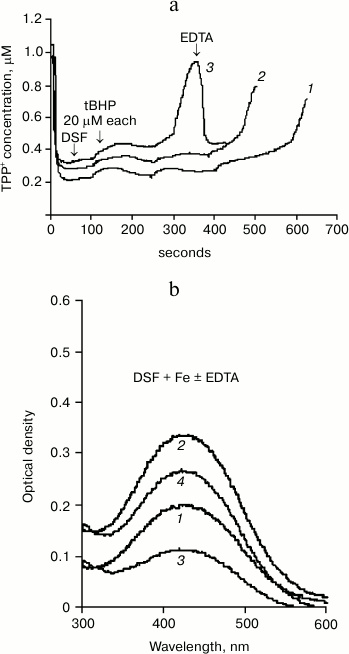
Figure 1a shows data on the effects of desferal (DSF) on the development of oxidative stress obtained using our method. One can see that Δψ changes caused by successive tBHP additions (at final concentration of 20 μM each) in the presence of 200 μM DSF (curve 2) differ only slightly from the control (curve 1), although a minor prooxidant effect of DSF can be observed.
Fig. 1. Effect of DSF on tBHP-induced MPTP opening. a) Changes in membrane potential caused by successive tBHP additions (at final concentration 20 μM each) in the control (1) and in the presence of 200 (2) and 500 μM (3) desferal. Addition of 50 μM EDTA restores the membrane potential. Incubation medium: 75 mM sucrose, 10 mM MOPS, 1.5 mM phosphate, 5 mM KCl, pH 7.4, 4 mM pyruvate. b) Absorption spectra (with 435-nm maximum) of DSF (100 μM) complex with iron at concentration 50 (1) and 100 μM (2); effect of 50 μM EDTA on the formation of DSF complex with iron at FeSO4 concentration of 50 (3) and 100 μM (4). Incubation medium is the same, but without substrate and phosphate.
However, addition of 500 μM DSF to mitochondria causes a distinct prooxidant effect (Fig. 1a, curve 3). This amount of DSF is close to the amount used in medical treatment. EDTA (50 μM) restores Δψ decreased in the presence of tBHP and DSF.
It should be noted that EDTA can lower the concentration of reduced iron more efficiently and with no known side effects, as indicated by the spectral data presented in Fig. 1b.
DSF forms a brown-colored complex with iron having an absorption maximum at 435 nm. Figure 1b shows the spectra of desferal added to incubation medium without mitochondria in the presence of 50 (curve 1) and 100 μM (curve 2) FeSO4. The next curves (3 and 4) show the spectra of DSF complex with iron formed in EDTA-containing medium with the same iron concentrations. Figure 1b shows that EDTA, an iron chelator, significantly reduces the absorption of the DSF complex with iron due to partial binding of Fe2+. This method can be used to estimate the amount of DSF-bound iron under different incubation conditions as well as in the presence of biologically active compounds.
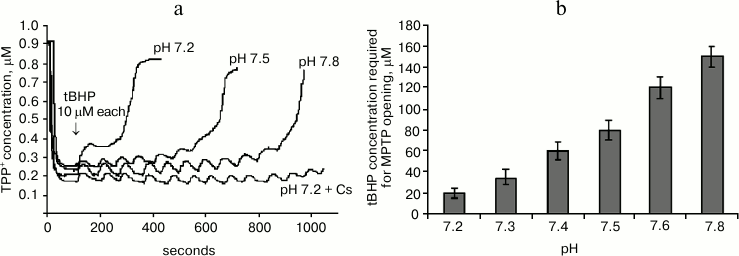
Figure 2a shows the effect of pH of the mitochondrial incubation medium on tBHP-induced MPTP opening. It shows mitochondrial membrane potential changes after successive tBHP additions at final concentration 10 μM each at different values of the pH of the medium. It can be seen that increasing the pH causes the number of additions leading to MPTP opening to increase many-fold. At pH of about 7.8 the number of tBHP additions is almost the same as when adding cyclosporin A.
Fig. 2. Effect of pH of the incubation medium on tBHP-induced MPTP opening. a) Original curves of mitochondrial membrane potential changes caused by successive tBHP additions at final concentration of 10 μM each at different pH values of the incubation medium and in the presence of cyclosporin A (Cs). b) Dependence of tBHP concentration required for MPTP opening on pH of the incubation medium. Incubation medium: 75 mM sucrose, 10 mM MOPS, 1.5 mM phosphate, 5 mM KCl, 4 mM pyruvate.
Figure 2b shows the data from experiments where the same method was used to pass the entire studied pH range three times in increments of 0.1 pH unit. This figure shows mitochondrial resistance to tBHP increases dramatically when approaching pH 7.8.
It is also important to note that the number of tBHP additions in the presence of previously introduced cyclosporin A (2 μM) at any given pH value to be the same (data not shown), and its effect on the number of tBHP additions was similar to the effect of medium alkalization to pH 7.8.
DISCUSSION
Experiments with desferal have shown iron (Fe2+) to bind with EDTA far better than with DSF. It should be added that (Fe2+) affinity to EDTA is several orders of magnitude higher than its affinity to DSF. Although EDTA also has a high affinity to aluminum, in our conditions EDTA can be considered as a specific iron chelator. EDTA can be used to treat pathologies with excessive iron accumulation, first in model experiments with animals. It is likely to bind iron in the bloodstream, thereby reducing its entry into cells. The most interesting result of this study was obtained in the experiments carried out to test the efficiency of alkalization for treating several pathologies caused by oxidative stress. We showed that a slight increase in the pH of the incubation medium increases dramatically the amount of tBHP that can be added before opening of the MPTP when added to incubation medium with isolated liver mitochondria. At the same time, the amount of tBHP added to mitochondria without MPTP opening under the same conditions but in the presence of cyclosporin A was practically the same. Thus, slight alkalization of incubation medium has no effect on tBHP transport, but it delays MPTP opening.
This conclusion causes absolutely no doubts on the model of liver mitochondria suspension. In all the numerous samples, pH increase of at least one-tenth of a pH unit and more caused both Δψ increase and increase in the number of tBHP additions before pore opening. A previously described [9] method of non-parametric statistics, “rule of signs”, shows very high probability of our conclusions being true, if the sign of the effect is repeated in all seven of the conducted independent experiments (see tables in the description of this method in [14]). Experiments described in this paper are much more reliable as not only the general direction of the effects, but also their values are similar.
All these data show the mitochondrial pathology model developed in our studies to be useful for solving a variety of medical and biological problems. This is due to the combination of its following features: 1) MPTP properties depend on many conditions. The data published in [14] clearly illustrate that not only changes in MPTP activators, but also increased incubation time with them change the properties of the pore (even if pH of the incubation medium does not change), in particular, its sensitivity to cyclosporin A. In our experiments, MPTP opening resulted from the increase in prooxidant tBHP concentration, and it depended only on tBHP concentration; 2) experiments were carried out on mitochondria with the highest Δψ, i.e. on intact mitochondria. Lipophilic cations of TPP+-type are known to accumulate electrophoretically, i.e. they are concentrated in mitochondria with the highest Δψ. A more detailed discussion of this property and relevant references can be found in the section “Some Methodological Aspects of Experiments with Mitochondria…” in [15]; 3) mitochondria were incubated under conditions close to physiological. The pH of the incubation medium was ~7.4, which is close to the average pH of blood. In the main experiments, mitochondria were isolated without washing and addition of such stabilizers as EGTA, EDTA, BSA, and others.
E. Cayce stated that a small increase in pH (alkalization) of body fluids will provide a cure for a number of serious pathologies (it could be measured by pH changes in blood, lymph, urine, saliva [12]). He suggested measuring saliva pH with test strips. The mitochondrial model used in our experiments enables further research in this direction. For example, it seems interesting and important to understand the mechanism of the strong effect of a small increase in pH on MPTP induction by the prooxidant tBHP.
The methods developed by E. Cayce are described not only in many of his own publications, but also in a number of articles published by doctors, healers, and patients working together with him in his institute (USA). We have chosen to refer to the publication that is the most accessible for the study and application of his methods [12].
We express heartfelt gratitude to Vladimir P. Skulachev and Innocent V. Skulachev for various help during the research.
REFERENCES
1.Kowaltowski, A. J., and Vercesi, A. E. (1999)
Free Radic. Biol. Med., 26, 463-471.
2.Papa, S., and Skulachev, V. P. (1997) Mol. Cell
Biochem., 174, 305-319.
3.Haworth, R. A., and Hunter, D. R. (2000) J.
Bioenerg. Biomembr., 32, 91-96.
4.Kowaltowski, A. J., Netto, L. E., and Vercesi, A.
E. (1998) J. Biol. Chem., 273, 12766-12769.
5.Vercesi, A. E., Kowaltowski, A. J., Grijalba, M.
T., Meinicke, A. R., and Castilho, R. F. (1997) Biosci Rep.,
17, 43-52.
6.Cervinkova, Z., Krivakova, P., Labajova, A.,
Rousar, T., Lotkova, H., Kucera, O., Endlicher, R., Cervinka, M., and
Drahota, Z. (2009) Arch. Toxicol., 83, 63-72.
7.Endlicher, R., Krivakova, P., Rauchova, H.,
Nuskova, H., Cervinkova, Z., and Drahota, Z. (2009) Physiol.
Res., 58, 685-692.
8.Cao, L., Waldon, D., Teffera, Y., Roberts, J.,
Wells, M., Langley, M., and Zhao, Z. (2013) Anal. Bioanal.
Chem., 405, 2635-2642.
9.Fedotcheva, N. I., and Mokhova, E. N. (2013)
Biochemistry (Moscow), 78, 75-79.
10.Pardo Andreu, G. L., Inada, N. M., Vercesi, A.
E., and Curti, C. (2009) Arch. Toxicol., 83, 47-53.
11.Devanur, L. D., Evans, R. W., Evans, P. J., and
Hider, R. C. (2008) Biochem. J., 409, 439-447.
12.Bolton, B. (compiler) (2004) An Edgar Cayce
Encyclopedia of Foods for Health and Healing (McCarey, W. A., ed.)
A.R.E. Press, Virginia Beach, Virginia.
13.Fernandez, V., and Winkelmann, G. (2005)
Biometals, 18, 53-62.
14.Sigel, S. (1956) Nonparametric Statistics in
Science and Behavior, N.Y.
15.Broekemeier, K. M., and Pfeiffer, D. R. (1995)
Biochemistry, 34, 16440-16449.
16.Mokhova, E. N. (2012) Biophys. Rev. Lett.,
7, 1-13.