Calix[4]arene C-90 Selectively Inhibits Ca2+,Mg2+-ATPase of Myometrium Cell Plasma Membrane
T. A. Veklich1*, A. A. Shkrabak1, N. N. Slinchenko1#, I. I. Mazur1, R. V. Rodik2, V. I. Boyko2, V. I. Kalchenko2, and S. A. Kosterin1
1Palladin Institute of Biochemistry, National Academy of Sciences of Ukraine, Leontovicha str. 9, 01601 Kiev, Ukraine; fax: +38 (044) 279-6365; E-mail: veklich@biochem.kiev.ua2Institute of Organic Chemistry, National Academy of Sciences of Ukraine, Murmanskaya str. 5, 02660 Kiev, Ukraine; fax: +38 (044) 573-2643; E-mail: vik@ioch.kiev.ua
* To whom correspondence should be addressed.
# Deceased.
Received November 19, 2013; Revision received January 31, 2014
The supramolecular compound calix[4]arene C-90 (5,11,17,23-tetra(trifluoro)methyl(phenylsulfonylimino)-methylamino-25,26,27,28-tetrapropoxycalix[4]arene) is shown to efficiently inhibit the ATP hydrolase activity of Ca2+,Mg2+-ATPase in the myometrium cell plasma membrane fraction and also in a preparation of the purified enzyme solubilized from this subcellular fraction. The inhibition coefficient I0.5 values were 20.2 ± 0.5 and 58.5 ± 6.4 µM for the membrane fraction and the solubilized enzyme, respectively. The inhibitory effect of calix[4]arene C-90 was selective comparatively to other ATPases localized in the plasma membrane: calix[4]arene C-90 did not influence the activities of Na+,K+-ATPase and “basal” Mg2+-ATPase. The inhibitory effect of calix[4]arene C-90 on the Ca2+,Mg2+-ATPase activity was associated with the cooperative action of four trifluoromethylphenylsulfonylimine (sulfonylamidine) groups oriented similarly on the upper rim of the calix[4]arene macrocycle (the calix[4]arene “bowl”). The experimental findings seem to be of importance for studies, using calix[4]arene C-90, of membrane mechanisms of regulation of calcium homeostasis in smooth muscle cells and also for investigation of the participation of the plasma membrane Ca2+-pump in control of electro- and pharmacomechanical coupling in myocytes.
KEY WORDS: Ca2+,Mg2+-ATPase, plasma membrane calcium pump, smooth muscle cells, myometrium, calix[4]arenes, sulfonylamidinesDOI: 10.1134/S0006297914050058
Abbreviations: PM, plasma membrane; SMC, smooth muscular cells.
Calcium ions (Ca2+) play a fundamental role in providing
electro- and pharmacomechanical coupling in smooth muscles including
those of the myometrium [1]. The Ca2+
concentration ([Ca2+]i) in relaxed smooth muscle
cells (SMC) is about 100 nM, which is approximately 104
times lower than in the extracellular medium
(~10–3 M). Extracellular Ca2+ is the
most important factor responsible for activation of SMC contraction. On
excitement, the [Ca2+]i value increases up to at
least several hundreds nM, but not higher than 1 µM.
Such increase in the calcium concentration in myocytes is due to its
passive entry into them through calcium channels of the plasma membrane
(PM) and also due to ejection of this cation from the major
intracellular calcium depot, the sarcoplasmic reticulum;
Ca2+ is released also from mitochondria [2, 3]. The relaxation
(“termination”) of the calcium signal is realized by the
functioning of active Ca2+-transporting systems,
Mg2+,ATP-dependent calcium pumps of PM and sarcoplasmic
reticulum (Ca2+,Mg2+-ATPases)
(thapsigargin-insensitive and thapsigargin-sensitive, respectively),
the Na+-Ca2+-exchanger of PM, and also
Ca2+-uniporter of mitochondria [4]. On
the whole, changes in [Ca2+]i are a result of
superposition of functioning of the membrane-related systems of passive
and active transport of Ca2+.
In uterine myocytes the Mg2+,ATP-dependent calcium pump of the PM having high affinity for Ca2+ (the activation constant KCa2+ by Ca2+ is 0.3-0.6 µM) performs the most important role in the control of [Ca2+]i [5, 6] because the affinity for Ca2+ for the Na+-Ca2+-exchanger of PM (KCa2+ ≈ 7-50 µM) is significantly lower than that for the calcium pump. Kinetic analysis of the trans-sarcolemma exchange of Ca2+ has shown that the functioning of this pump on the background of the continuous entry of a slow diffuse calcium inflow into non-excited uterine myocytes (10–15-10–14 mol Ca2+/cm2 per s) is quite sufficient for long-term stable maintenance of [Ca2+]i in the myoplasm at the level of <10–6 M. Thus, the calcium pump of PM is the most important element of the general control mechanism of the basal tonus of the myometrium. If this pump did not exist, then, on taking into account the saturation of intracellular structures with Ca2+, myocytes would acquire a state of a steady contracture after approximately 100 contractions activated by entry into SMC of extracellular Ca2+ as a result of a series of depolarizing pulses [7].
Selective inhibitors of energy-dependent Ca2+-transporting systems are extremely necessary for studies on the role of these systems in the control of cellular functions under normal and pathological conditions. In particular, o-vanadate and eosin are nonspecific inhibitors of the Ca2+,Mg2+-ATPase of PM. However, o-vanadate inhibits not only Ca2+,Mg2+-ATPase and Na+,K+-ATPase of PM, but it also inhibits indirectly the system of Na+-Ca2+-exchange localized in the same subcellular structure (as a result of inhibition of the sodium pump activity and, respectively, a decrease in the transmembrane sodium gradient). Eosin inhibits Ca2+- and Na+-transporting ATP-hydrolases of PM and also Ca2+-transporting ATP-hydrolase of the sarco(endo)plasmic reticulum [8]. Relatively selective new inhibitors of the calcium pump of PM, the caloxin peptides, have been described recently [9]. But low molecular weight selective inhibitors of the PM Ca2+,Mg2+-ATPase are unknown. However, it is obvious that the search for them is extremely important for clinical practice because nontoxic compounds capable of selective blocking functioning of the Mg2+,ATP-dependent calcium pump of PM are promising for creation of pharmaceuticals regulating the tonus and contractility of the uterus under pathological situations (uterine inertia, uterine hypotonus, uterine bleeding, etc.).
In the present work, we present findings indicating that the compound calix[4]arene C-90 can selectively (comparatively to other ATP-hydrolase systems) inhibit Ca2+,Mg2+-ATPase of the myometrium cell PM.
MATERIALS AND METHODS
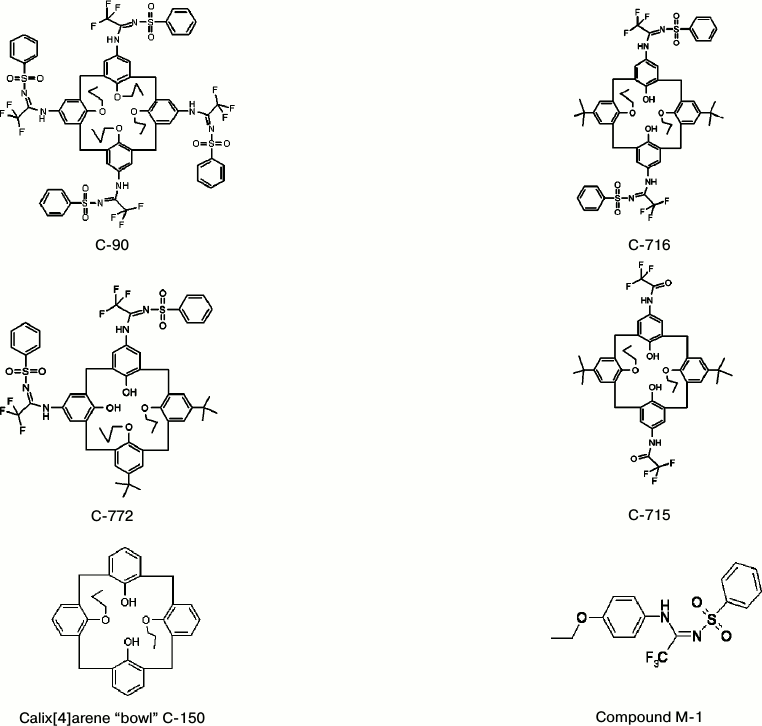
Calix[4]arenes. Calix[4]arenes were synthesized in the Department of Phosphorane Chemistry, Institute of Organic Chemistry, National Academy of Sciences of Ukraine (Chief – Correspondent Member of the National Academy of Sciences of Ukraine Prof. V. I. Kalchenko). Calix[4]arene C-90 was synthesized as described earlier [10]. The structure of the synthesized calix[4]arenes was confirmed by infrared and NMR spectroscopy.
The following compounds were used in experiments:
– calix[4]arene C-90 — (5,11,17,23-tetra(trifluoro)methyl(phenylsulfonylimino)-methylamino-25,26,27,28-tetrapropoxycalix[4]arene), and also its analogs:
– calix[4]arene C-715 — 5,17-di(trifluoro)acetamido-11,23-di-tret-butyl-25,27-dipropoxycalix[4]arene;
– calix[4]arene C-716 — 5,17-di(trifluoro)methyl(phenylsulfonylimino)-methylamino-11,23-di-tret-butyl-25,27-dipropoxycalix[4]arene;
– calix[4]arene C-772 — 5,11-di(trifluoro)methyl(phenylsulfonylimino)-methylamino-17,23-di-tret-butyl-26,27-dipropoxycalix[4]arene;
– calix[4]arene C-150 — 25,27-dipropoxycalix[4]arene (the calix[4]arene “bowl” as it is);
– the compound M-1 — N-(4-etoxyphenyl)-N′-(phenylsulfonyl)-trifluoromethylacetimidoamide (a structural element of the calix[4]arene C-90 molecule).
Structural formulas of studied compounds are presented in Fig. 1.
Fig. 1. Structural formulas of the studied calix[4]arenes and of compound M-1.
Biochemical studies on the influence of calix[4]arenes on the activity of Ca2+,Mg2+-ATPase in uterine SMC PM were performed in the Department of Muscle Biochemistry, Palladin Institute of Biochemistry, National Academy of Sciences of Ukraine (Chief – Correspondent Member of the National Academy of Sciences of Ukraine Prof. S. A. Kosterin).
The PM fraction of SMC was isolated from swine myometrium as described earlier in [11, 12]. The protein content in the membrane fraction was determined by the Bradford method [13].
The Ca2+,Mg2+-ATPase activity was determined in the PM fraction of the myometrium cells at 37°C in 0.4 ml of medium containing 3 mM ATP, 3 mM MgCl2, 0.95 mM CaCl2, 25 mM NaCl, 125 mM KCl, 1 mM EGTA, 20 mM Hepes-Tris buffer (pH 7.4), 1 mM NaN3 (an inhibitor of mitochondrial ATPase [14]), 1 mM ouabain (an inhibitor of Na+,K+-ATPase [15]), 0.1 µM thapsigargin (a selective inhibitor of Ca2+,Mg2+-ATPase of endo(sarco)plasmic reticulum [14]), and 0.1% digitonin (a factor of PM perforation [16]). The protein content of the membrane fraction in the sample was 20-30 µg. The incubation time was 5 min. The reaction was initiated by addition into the medium of an aliquot (50 µl) of the PM suspension and was stopped by addition to the reaction mixture of 1 ml of “stop” solution with the following composition: 1.5 M sodium acetate, 3.7% formaldehyde, 14% ethanol, 5% TCA (pH 4.3 at 8°C). The Ca2+,Mg2+-ATPase activity was calculated by the difference between values of the ATPase activity in the absence and in the presence of calcium ions. In our experiments the mean value of the specific enzymatic activity of Ca2+,Mg2+-ATPase of the uterine myocyte PM was 3.4 ± 0.3 µmol Pi/mg protein per h (M ± m; n = 7).
A purified transporting Ca2+,Mg2+-ATPase solubilized with Triton X-100 was prepared from the swine myometrium cell PM using affinity chromatography on calmodulin-Sepharose 4B as described earlier [17]. The activity of the solubilized Ca2+,Mg2+-ATPase was determined at 37°C in 0.2 ml of standard incubation medium with the following composition: 50 mM Tris-HCl (pH 7.4), 125 mM KCl, 25 mM NaCl, 3 mM ATP, 3 mM MgCl2, 0.1 mM CaCl2, 0.1 mM EGTA, and 1.5-2.0 µg protein. The incubation time was 10 min. According to data of SDS-PAGE, the purified enzyme preparation contained two polypeptides with molecular weights of 130 and 205 kDa and was characterized by Ca2+-dependent autophosphorylation [17]. Based on the mean specific activity value of the solubilized Ca2+,Mg2+-ATPase (83.8 ± 7.4 µmol Pi/mg protein per h; M ± m; n = 7), this method allowed us to reach a 52-fold purification of the enzyme. Enzymatic activity of “basal” Ca2+-independent Mg2+-ATPase was not detected in the preparation of purified Ca2+,Mg2+-ATPase.
To detect “ouabain-sensitive” Na+,K+-ATPase activity, the “total” ATPase activity was determined in 0.4 ml of the standard incubation medium containing: 1 mM ATP, 3 mM MgCl2, 125 mM NaCl, 25 mM KCl, 1 mM EGTA, 20 mM Hepes-Tris buffer (pH 7.4), 1 mM NaN3, 0.1 µM thapsigargin, and 0.1% digitonin. The membrane fraction protein content in the sample was 20-30 µg. The incubation time at 37°C was 4 min. Under these conditions the “basal” Mg2+-ATPase activity was determined in the same medium but in the presence of 1 mM ouabain. The “ouabain-sensitive” Na+,K+-ATPase activity was calculated as the difference between values of the “total” ATPase activity and the “basal” Mg2+-ATPase activity. The mean value of the specific enzymatic activity of the “ouabain-sensitive” Na+,K+-ATPase of the uterine myocyte PM was 10.2 ± 0.7 µmol Pi/mg protein per h (M ± m; n = 7).
In all experiments the incubation medium without PM fragments or the purified Ca2+,Mg2+-ATPase preparation was used as control of non-enzymatic hydrolysis. As control on the endogenous inorganic phosphate (Pi) quantity in the membrane or enzyme preparation, incubation medium was used containing only PM fragments or the purified enzyme preparation in aqueous solution. The amount of the reaction product Pi was determined as described in [18].
The influence of calix[4]arenes and of compound M-1 on the activities of ATPases of PM was studied in the standard incubation medium described above; in all experiments initial concentrated solutions of calix[4]arenes (20 mM) in dimethylsulfoxide were used; at first, these solutions were diluted with water to final concentration of calix[4]arenes of 1 mM, and then aliquots of the diluted solutions of these compounds were introduced directly into the incubation medium. The final maximal concentration of dimethylsulfoxide in the incubation medium was 0.5%. We have shown earlier that dimethylsulfoxide in the concentration of 1% in the incubation medium does not influence the activity of the calcium pumps of PM, sarcoplasmic reticulum, and mitochondria of the myometrium cells [8]. The model compound M-1 was easily soluble directly in water.
In all experiments, the specific ATPase activity determined under standard conditions in the reaction medium without calix[4]arenes, compound M-1, or dimethylsulfoxide was taken as 100% (“the null point”).
The concentration dependences of the action of calix[4]arenes on the activity of Ca2+,Mg2+-ATPase were used to determine values of the inhibition coefficients I0.5 calculated using linearized Hill plots corresponding to the empiric equation: log[(A0 – A)/A] = –nH logI0.5 + nH log[I], where A0 and A are specific enzymatic activities in the absence (“the null point”) and in the presence of calix[4]arenes in the incubation medium in the concentration I. In the case of linearized plots, the typical value of the correlation coefficient r was 0.97-0.99.
Results of the studies were treated statistically with Student’s t-test. Kinetic and statistical calculations were performed using the MS Excel software.
Reagents used were as follows: ATP, Hepes, ouabain, and thapsigargin (Sigma, USA); digitonin (Serva, Germany); Tris-hydroxymethyl-aminomethane (Reanal, Hungary); EGTA (Fluka, Switzerland). Other reagents were of domestic production of analytical and chemical purity.
RESULTS AND DISCUSSION
In our experiments the mean value of the Ca2+,Mg2+-ATPase activity in the PM fraction was 3.4 ± 0.3 µmol Pi/mg protein per h (M ± m; n = 7), and the activity of a preparation of the purified solubilized Ca2+,Mg2+-ATPase was 83.8 ± 7.4 µmol Pi/mg protein per h (M ± m; n = 7) (in the last case “basal” Mg2+-ATPase activity was not detectable).
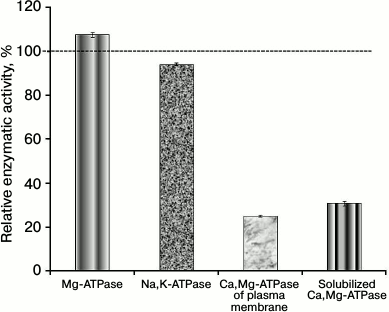
Our studies on the effect of calix[4]arenes on Mg2+-dependent ATP-hydrolase activities in the PM fraction of the myometrium cells have shown that calix[4]arene C-90 at the concentration of 100 µM inhibits the activity of Ca2+,Mg2+-ATPase to 25.1 ± 0.5% with relation to the control value taken as 100%. This calix[4]arene inhibited the activity of solubilized Ca2+,Mg2+-ATPase to 32.2 ± 2.3%. However, calix[4]arene C-90 (100 µM) had virtually no influence on the activities of Na+,K+-ATPase and “basal” Mg2+-ATPase, their residual activities in the presence of this compound were, respectively, 94.2 ± 0.6 and 107.7 ± 1.0% in relation to the control values (Fig. 2).
Fig. 2. Calix[4]arene C-90 (100 µM) inhibits Ca2+,Mg2+-ATPase activities of the plasma membrane fraction of myometrium cells and of the preparation of purified solubilized Ca2+,Mg2+-ATPase (M ± m, n = 5). Values of the specific enzymatic activities in the absence of calix[4]arene C-90 in the incubation medium are taken as 100%.
Thus, 100 µM calix[4]arene C-90 efficiently inhibited the Ca2+,Mg2+-ATPase activity in both the PM fraction of the uterine myocytes and the preparation of purified solubilized Ca2+,Mg2+-ATPase. It had almost no influence on the activities of other ATP-hydrolases of the PM fraction. Consequently, calix[4]arene C-90 selectively suppressed the Ca2+,Mg2+-ATPase activity of the PM fraction without influence on the activities of Na+,K+-ATPase and Mg2+-ATPase of the PM (we have shown also in independent experiments that calix[4]arene C-90 (100 µM) does not influence the accumulation of calcium ions in the myometrium mitochondria).
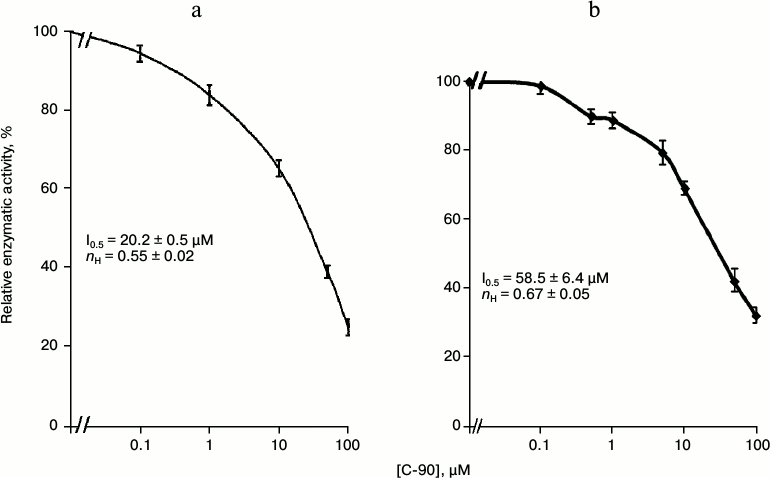
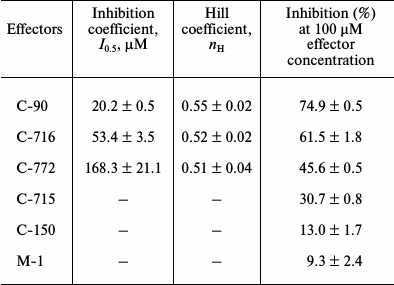
Then we studied the concentration dependence of the inhibitory effect of calix[4]arene C-90 (10–7-10–4 M) on the Ca2+,Mg2+-ATPase activity in the PM fraction (Fig. 3a). Calix[4]arene C-90 dose-dependently suppressed the Ca2+,Mg2+-ATPase activity, the inhibition coefficient I0.5 value was 20.2 ± 0.5 µM and the Hill coefficient nH value was 0.55 ± 0.02 (M ± m; n = 5) (table). Calix[4]arene C-90 also dose-dependently inhibited the ATP-hydrolase activity of the purified solubilized Ca2+,Mg2+-ATPase preparation (Fig. 3b), the I0.5 value was 58.5 ± 6.4 µM and the nH value was 0.67 ± 0.05 (M ± m; n = 5).
Fig. 3. Concentration dependences of the inhibitory effect of calix[4]arene C-90 on the enzymatic activities of Ca2+,Mg2+-ATPase of the plasma membrane fraction of myometrium cells (a) and of the purified solubilized Ca2+,Mg2+-ATPase preparation (b) (M ± m; n = 5). Value of the specific enzymatic activity in the absence of calix[4]arene C-90 in the incubation medium is taken as 100%.
Kinetic parameters of inhibitory effect of calix[4]arene C-90 and its structural analogs calix[4]arenes
C-716, C-772, C-715 as well as of the
calix[4]arene “bowl” C-150 and of the
model compound M-1 on specific enzymatic activity of
Ca2+,Mg2+-ATPase of plasma membrane of myometrium
cells (M ± m; n = 5)
Note: While investigating the concentration dependences of the influence
of calix[4]arenes on specific enzymatic activity of
Ca2+,Mg2+-ATPase, values of the inhibition
coefficients I0.5 and of Hill coefficients were
calculated using linearized Hill plots (values of correlation
coefficients were 0.98-0.99).
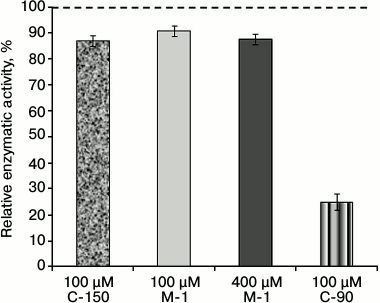
To determine the role of chemical groups of the calix[4]arene C-90 molecule in the inhibition of the activity of Ca2+,Mg2+-ATPase of the PM fraction, two model compounds were studied: the calix[4]arene “bowl” calix[4]arene C-150 and the compound M-1. Figure 1 shows that calix[4]arene C-150 is an unmodified calix[4]arene scaffold. The compound M-1 contains one phenolic fragment and a sulfonylamidine group analogous to those in the calix[4]arene C-90 molecule (Fig. 1). At 100 µM concentration, calix[4]arene C-150 insignificantly (by 13.0 ± 1.8%) decreased the Ca2+,Mg2+-ATPase activity in the PM fraction of the myometrium cells (M ± m; n = 5). The model compound M-1 at 100 µM concentration also suppressed Ca2+,Mg2+-ATPase slightly, by 9.3 ± 2.4%, and at 400 µM concentration (the C-90 molecule includes four M-1 fragments) it inhibited the Ca2+,Mg2+-ATPase activity by 12.5 ± 1.7% (M ± m; n = 5) (Fig. 4 and the table). However, calix[4]arene C-90 at 100 µM concentration inhibited efficiently (as compared to calix[4]arene C-150 and compound M-1) the Ca2+,Mg2+-ATPase activity in both the PM fraction of the uterine myocytes (by 75% with respect to the control) and the preparation of purified Ca2+,Mg2+-ATPase solubilized from PM (by 68% with respect to the control) (Figs. 2 and 3). Consequently, the inhibitory effect of calix[4]arene C-90 on the Ca2+,Mg2+-ATPase activity from the PM fraction (Figs. 2-4) is associated, first of all, with the cooperative influence of four sulfonylamidine groups spatially organized on the upper rim of the calix[4]arene scaffold and not with the action of the tetraphenol macrocycle as it is (the calix[4]arene “bowl” — calix[4]arene C-150), or with a possible effect of the separate sulfonylamidine residue (fragment M-1).
Fig. 4. Enzymatic activity of Ca2+,Mg2+-ATPase in the plasma membrane fraction of myometrium cells in the presence of calix[4]arenes C-90 and C-150 and also of the model compound M-1 (M ± m; n = 5). The specific enzymatic activity value in the absence of calix[4]arenes C-90 and C-150 and also of compound M-1 in the incubation medium is taken as 100%.
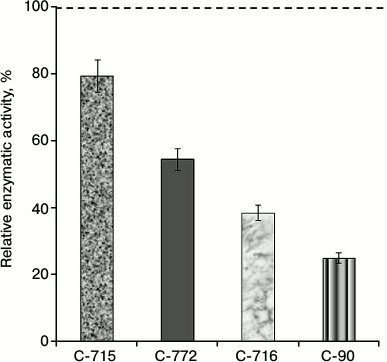
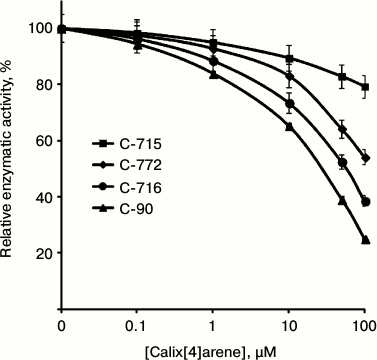
In the subsequent experiments we studied the influence of calix[4]arene C-90 structural analogs, that is calix[4]arenes C-715, C-716, and C-772 (Fig. 1), on the Ca2+,Mg2+-ATPase activity of PM. Obviously, the main structural features of these compounds are specific just for the upper rim of the calix[4]arene “bowl”. Thus, calix[4]arene C-716, in contrast to calix[4]arene C-90, contains only two phenylsulfonylamidine groups situated on the opposite phenolic residues of the calix[4]arene “bowl”, whereas on two other phenolic cycles tret-butyl residues are located instead of phenylsulfonylamidine groups. Calix[4]arene C-772, in contrast to calix[4]arene C-90, also has only two phenylsulfonylamidine groups, but they are located on the neighboring phenolic residues of the calix[4]arene “bowl”, whereas on the two other phenolic cycles, as in the case of calix[4]arene C-716, tret-butyl residues are located instead of phenylsulfonylamidine groups. Finally, calix[4]arene C-715 has the simplest structure: compared to calix[4]arene C-716, it lacks phenylsulfonyl residues. That is, trifluoromethylcarbonylamino groups are located on its upper rim instead of trifluoromethyl(phenylsulfonylimino)methylamino groups (Fig. 1). Our studies revealed that calix[4]arenes used at 100 µM concentration suppressed the activity of Ca2+,Mg2+-ATPase of PM, on average, by 20% (C-715), by 45% (C-772), and by 61% (C-716), whereas calix[4]arene C-90 suppressed the Ca2+,Mg2+-ATPase activity by 75% (as compared to the control value) (Fig. 5). None of these calix[4]arenes influenced the activities of Na+,K+-ATPase and “basal” Mg2+-ATPase. In our subsequent experiments, we studied concentration (10—7 to 10—4 M) dependences of action of the above-mentioned calix[4]arenes on the activity of Ca2+,Mg2+-ATPase of PM. All these compounds were shown to dose-dependently suppress this activity (Fig. 6). The I0.5 values were 168.3 ± 21.1 and 53.4 ± 3.5 µM, respectively, for calix[4]arenes C-772 and C-716, whereas for calix[4]arene C-90 the I0.5 value was 20.2 ± 0.5 µM. It is impossible to correctly determine the inhibition coefficient I0.5 for calix[4]arene C-715 because of its weak inhibitory influence on the activity of Ca2+,Mg2+-ATPase (Figs. 5 and 6 and the table).
Fig. 5. Enzymatic activity of Ca2+,Mg2+-ATPase in the plasma membrane fraction of myometrium cells in the presence of calix[4]arene C-90 and of its structural analogs: calix[4]arenes C-716, C-772, and C-715 (M ± m; n = 5). The specific enzymatic activity value in the absence of calix[4]arenes in the incubation medium is taken as 100%.
Fig. 6. Concentration dependences of the inhibitory effect of calix[4]arene C-90 and of its structural analogs on the activity of Ca2+,Mg2+-ATPase in the plasma membrane fraction of myometrium cells (M ± m; n = 5). The specific enzymatic activity in the absence of calix[4]arenes in the incubation medium is taken as 100%.
Thus, to efficiently inhibit the Ca2+,Mg2+-ATPase activity of PM (Figs. 2-5), the calix[4]arene molecule has to have four phenylsulfonylamidine groups (calix[4]arene C-90) on the upper rim of the macrocycle (Fig. 1). However, it should be noted that the inhibitory effects of the studied calix[4]arenes (Fig. 1) on the Ca2+,Mg2+-ATPase activity of PM also depended on the number and mutual location of phenylsulfonylamidine groups on the upper rim of the calix[4]arene “bowl” (Figs. 5 and 6).
The activity of Ca2+,Mg2+-ATPase of PM is a biochemical manifestation of functioning of the Mg2+,ATP-dependent calcium pump that pumps calcium ions from the myoplasm into the extracellular medium [1, 2, 5, 6, 19, 20]. Considering the kinetic parameters of Ca2+,Mg2+-ATPase of PM and the inhibitory effect of calix[4]arene C-90 on its activity that we determined earlier, we created (in a stable regimen) a model of the influence of this inhibitor on the “basal” concentration of calcium ions ([Ca2+]i) in an unexcited uterine myocyte. According to results of the modeling, the inhibitory effect of calix[4]arene C-90 on Ca2+,Mg2+-ATPase of PM has to lead to an increase in [Ca2+]i, and the [Ca2+]i dependence on the C-90 concentration is biphasic: within the calix[4]arene concentration range of 1-25 µM, this compound promotes a moderate increase in [Ca2+]i in unexcited myocytes (approximately to 150-200 nM), whereas at concentration of C-90 > 25 µM the [Ca2+]i value increases exponentially with an increase in the calix[4]arene concentration [21]. In this regard, it should be noted that in preliminary experiments performed in our laboratory by L. G. Babich and S. G. Shlykov with the Ca2+-sensitive fluorescent probe Fluo-4AM, it was found that the Ca2+,Mg2+-ATPase inhibitor calix[4]arene C-90 (100 µM) induced an increase in the “basal” [Ca2+]i and decelerated the relaxation of the oxytocin-induced (100 nM) calcium signal in uterine myocytes.
We suppose that our experimental data on the selective and sufficiently high affinity (I0.5 ≈ 20 µM) inhibitory effect of calix[4]arene C-90 on the activity of transport Ca2+,Mg2+-ATPase of PM can be used for further elucidation of mechanisms regulating Ca2+ concentration in SMC and of the role of this pump in control of electromechanical coupling in myocytes.
Taking into account the nontoxic nature of calixarenes [22], we think that our data are promising for use in practice of calix[4]arene C-90 as a “soft” stimulator of uterine contractility.
This work was supported by the State Registration Projects Nos. 0110U000988, 0110U005970, 0110U005971, 0111U007135, 0112U004262, and 0113U007570.
REFERENCES
1.Koide, M., Nystoriak, M. A., Brayden, J. E., and
Wellman, G. C. (2011) Acta Neurochir. Suppl., 110, Pt. 1,
145-150.
2.Kosterin, S. A. (1990) Calcium Transport in
Smooth Muscles [in Russian], Naukova Dumka, Kiev.
3.Veklich, T. (2002) Annales Universitatis Mariae
Curie-Sclodowska, XV, No. 39, 427-431.
4.Gonzales, A. L., Amberg, G. C., and Earley, S.
(2010) Am. J. Cell Physiol., 299, 279-288.
5.Carafoli, E., and Brini, M. (2000) Curr. Opin.
Chem. Biol., 4, 152-156.
6.Kosterin, S. O. (2003) Neurophysiology,
35, 215-228.
7.Cartwright, E. J., Oceandy, D., Austin, C., and
Neyses, L. (2011) Sci. China Life Sci., 54, 691-698.
8.Kosterin, S. A., Bratkova, N. F., Babich, L. G.,
Shinlova, O. P., Slinchenko, N. N., Shlykov, S. G., Zimina, V. P.,
Rovents, N. A., and Veklich, T. A. (1996) Ukr. Biokhim. Zh.,
68, 50-61.
9.Szewczyk, M. M., Pande, J., and Grover, A. K.
(2008) Pflugers Arch. Eur. J. Physiol., 456, 255-266.
10.Rodik, R. V., Boyko, V. I., Danylyuk, O. B.,
Suwinska, K., Tsymbal, I. F., Slinchenko, N. V., Babich, L. G.,
Shlykov, S. O., Kosterin, S. O., Lipkowski, J., and Kalchenko, V. I.
(2005) Tetrahedron Lett., 46, 7459-7462.
11.Veklich, T. A., and Kosterin, S. A. (2005)
Ukr. Biokhim. Zh., 77, 66-75.
12.Kondratyuk, T. P., Bychenyuk, S. F., Pishchepa,
A. A., Babich, L. G., Kursky, M. D., and Osipenko, A. A. (1986) Ukr.
Biokhim. Zh., 58, 50-56.
13.Bradford, M. M. (1976) Anal. Biochem.,
72, 248-282.
14.Flynn, E. R. M., Bradley, K. N., Muir, T. C., and
McCarron, J. G. (2001) J. Biol. Chem., 276,
36411-36418.
15.Robinson, J. D. (1983) Biochim. Biophys.
Acta, 727, 63-69.
16.Veklich, T. A., Kosterin, S. A., and Shinlova, O.
P. (2002) Ukr. Biokhim. Zh., 74, 42-48.
17.Slinchenko, N. N., Lyubakovskaya, L. A., Kursky,
M. D., and Sopel, L. V. (1990) Ukr. Biokhim. Zh.,
62, 60-65.
18.Rathbun, W. B., and Betlach, M. V. (1969)
Anal. Biochem., 28, 436-445.
19.Carafoli, E. (2011) Sci China Life Sci.,
54, 686-690.
20.Kosterin, S. A., and Burdyga, F. V. (1991)
Usp. Sovr. Biol., 113, 485-506.
21.Veklich, T. O., Shkrabak, O. A., Mazur, Y. Y.,
Rodik, R. V., Boiko, V. I., Kalchenko, V. I., and Kosterin, S. O.
(2013) Ukr. Biokhim. Zh., 85, 20-29.
22.Rodik, R. V. (2012) Ukr. Biokhim. Zh.,
84, 5-15.