Analysis of Extracellular Vesicles Using Magnetic Nanoparticles in Blood of Patients with Acute Coronary Syndrome
M. S. Vagida1#, A. Arakelyan2#, A. M. Lebedeva1*, J.-Ch. Grivel2, A. V. Shpektor1, E. Yu. Vasilieva1, and L. B. Margolis2
1Moscow State University of Medicine and Dentistry, Laboratory of Atherothrombosis, 109240 Moscow, Russia; E-mail: asya.lebedev@mail.ru2Section on Intercellular Interactions, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, 20892, Bethesda, Maryland, USA
# These authors contributed equally to this work.
* To whom correspondence should be addressed.
Received September 9, 2015; Revision received December 15, 2015
Extracellular vesicles (EVs) are released from various cell types and play an important role in intercellular interactions. In our study, we investigated abundance of individual EVs in patients with acute forms of ischemic heart disease. Previously, we developed an approach for individual analysis of EVs conjugated with magnetic nanoparticles (MNPs), which was applied in the current study for analyzing phenotypic composition of EVs (by staining for markers CD31, CD41a, and CD63). EVs were isolated using fluorescently labeled MNPs containing anti-CD31, CD41a, or CD63 antibodies and analyzed by combining fluorescently labeled anti-CD41a and CD63, CD31 and CD63, or CD41a and CD31 antibodies, respectively. EVs were analyzed in 30 individuals: 17 healthy volunteers and 13 patients with acute coronary syndrome (ACS). Six and seven ACS patients were with acute myocardial infarction and unstable angina, respectively. It was found that patients with ACS and healthy volunteers contained a dominant subset of EVs expressing surface CD41a antigen, suggesting that they originated from platelets. In addition, the total number of EVs isolated using either of the surface markers examined in our study was higher in patients with ACS compared to healthy volunteers. The subgroup of patients with acute myocardial infarction was found to contain significantly higher number of blood EVs compared to the control group. Moreover, increased number of EVs in patients with ACS is mainly due to the increased number of EVs in the subset of EVs bearing CD41a. By analyzing individual EVs, we found that plasma of patients with ACS, particularly upon developing of myocardial infarction, contained dominant platelet-derived EVs fraction, which may reflect activation of platelets in such patients.
KEY WORDS: extracellular vesicles, platelets, acute coronary syndrome, flow cytometryDOI: 10.1134/S0006297916040088
In recent years, so-called extracellular vesicles (EVs) have attracted much attention. EVs are formed by lipid bilayer membrane, which can be released into the environment by various cells. These vesicles play an important role in intercellular interactions and can target different cells and transfer to them “packed” proteins and lipids as well as miRNA that are typical for the cells of origin [1, 2]. It seems that EV exchange between cells is of great importance both in normal settings and in pathologies [3, 4]. For example, it was shown that EVs take part in regulating blood clotting [5-9], can serve as novel tumor biomarkers, etc. [10, 11].
Overall analysis of human EVs without individual characterization of each EVs subset revealed that they typically bear tetraspanins such as CD63, which is expressed by numerous cell types and is involved in EV formation [12]. Moreover, it was demonstrated that two other proteins are often detected on the surface of human EVs in blood: CD41a, platelet surface glycoprotein receptor IIb/IIIa, and CD31 belonging to adhesion proteins typical of but not specific to vascular endothelium [6, 13-18]. Extracellular vesicles can indicate not only their own origin, but also report on the cell status [19]. In particular, by examining the overall pool of the vesicles without their individual characterization, it was demonstrated that the number and composition of microparticles (vesicles of more than 300 nm in diameter) change upon exacerbations of ischemic heart disease, particularly during acute myocardial infarction [20, 21]. This disease is characterized by growth and rupture of atherosclerotic plaques in the wall of coronary arteries followed by formation of thrombi occluding the vascular lumen [22].
Despite obvious heterogeneity of EVs reflecting diversity of the cells releasing them, in the majority of available publications EVs were examined as a whole pool. For instance, in the reports mentioned above, exosome fractions representing the smallest vesicles comprising >90% of total EVs were not analyzed during ischemic heart disease [23]. Various biochemical assays used in these works did not allow for characterization of antigen composition of individual EVs. To do this, an analysis is required similar to a flow cytometry of cells [24]. However, a standard flow cytometer does not allow examining EVs due to their low light scattering properties. Moreover, if EVs were stained with various fluorescent antibodies, then during flow cytometry it might hinder discrimination between stained EVs and free antibodies or their aggregates having similar size.
We were able to solve these issues by developing a new approach for characterizing separate small vesicles and evaluating various antigens on their surfaces. The current study was aimed to characterize EVs from the blood of healthy volunteers and patients with acute ischemic heart disease using a new approach for analysis of individual vesicles, including the smallest ones.
MATERIALS AND METHODS
Patients and healthy controls. Thirty persons including 17 healthy volunteers and 13 patients admitted to the Davydovskiy Moscow City Clinical Hospital diagnosed with acute coronary syndrome (ACS) were studied. Among patients with acute forms of ischemic heart disease (IHD), six were with acute myocardial infarction (AMI) and seven with unstable angina (UA). Acute coronary syndrome, acute myocardial infarction, and unstable angina were diagnosed according to the generally accepted criteria [22, 25, 26]. All patients received a standard dual antiplatelet therapy (acetylsalicylic acid and clopidogrel), and the majority received anticoagulant therapy during transportation to the hospital (1-2 h before collection of blood samples) in accordance with the current international recommendations. Patients with infectious diseases, neoplasms, cardiogenic shock, or thrombolytic therapy were excluded from the study. All participants provided a signed voluntary informed consent declaration.
Samples of peripheral venous blood were collected from patients with IHD and healthy volunteers into test tubes containing 3.2% sodium citrate solution (Sarstedt, Germany) with the first 5 ml-sample not being used to avoid isolation of EVs released by platelets due to their activation during venipuncture. Blood samples were collected from patients within the first 24 h after admission to the hospital and prior to percutaneous coronary intervention. Blood samples were centrifuged for 15 min at 3000g to obtain platelet-poor plasma (PPP) followed by freezing at –80°C.
Isolation of EVs. In the current study, we used a technique for isolation and analysis of individual EVs that was previously reported by us [27], with small modifications. Magnetic separation was done by using nanoparticles coupled with antibodies against CD31, CD41a, and CD63 (Biolegend, USA). Briefly, 15-nm iron oxide magnetic nanoparticles (MNPs) coated with carboxyl groups (Ocean NanoTech, USA) were coupled with purified monoclonal antibodies against human CD31, CD41a, and CD63. For this, 1 mg of MNPs were incubated in 400 µl of activation buffer containing 1.7 mM 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 0.76 mM N-hydroxysuccinimide sulfate for 10 min at room temperature. After activation, MNPs were supplemented with 400 µl of coupling buffer followed by immediate addition of 1 mg of purified antibodies. After 2 h of incubation in a thermomixer at room temperature with gentle mixing, the reaction was stopped by adding 10 µl of quenching solution followed by two washouts by using a magnetic separator (SuperMAG-01; Ocean NanoTech) at 4°C. MNPs conjugated to antibodies were resuspended in 2 ml of storage buffer and kept at 4°C; the final concentration of iron oxide was 0.5 mg/ml.
For subsequent flow cytometry analysis, MNPs coupled with antibodies were stained with fluorescent Alexa Fluor 488-labeled Fab-fragment of IgG of goat antibodies against mouse immunoglobulins (Zenon mouse IgG labeling reagent; Life Technologies, USA) for 20 min at room temperature with gentle mixing (6 µl Fab-fragment per 60 µl magnetic particles). After incubation, the mixture was applied to phosphate buffer pre-wetted 100-kDa columns (Nanosep, USA) and centrifuged at 1100g for 5 min, followed by washing with 200 µl of phosphate buffer. The resulting antibody-coupled and Fab-Alexa Fluor 488-labeled MNPs, free of unbound Fab-fragment, were resuspended in the initial volume using filtered phosphate-buffered saline (Gibco, Life Technologies).
These MNPs (labeled with Fab-fragments and conjugated with antibodies) were incubated with a thawed PPP sample at a ratio of 100 µl PPP per 60 µl MNPs for 1 h at 4°C. The solution of blocking agent (Molecular Probes, Life Technologies) was added at 2.5% concentration to block unspecific labeling of following stain. Then, a combination of fluorescent monoclonal antibodies against various cell surface antigens of interest was added to the solution. Isotype-matched fluorescently labeled antibodies were used to assess specificity of recognition. We used the following combinations of monoclonal antibodies against EV-characteristic surface proteins: for CD31-conjugated MNPs – anti-CD41a-APC (BD Bioscience, USA) and anti-CD63-PE (Biolegend); for CD41a-conjugated MNPs – anti-CD31-AlexaFluor® 647 (Biolegend) and anti-CD63-PE (Biolegend); for CD63-conjugated MNPs – anti-CD31-PE (Biolegend) and anti-CD41a-APC (BD Bioscience). In addition, the following isotype-match antibodies were used as a control: Alexa Fluor 647-mouse IgG1κ (Biolegend), PE-mouse IgG1κ (Biolegend), APC-mouse IgG1κ (BD), Alexa Fluor 488-mouse IgG1κ (eBioscience, USA). A suspension was incubated for 20 min in the dark followed by isolating MNP–EV–detection antibody complex by applying a strong magnetic field in a MACS® magnetic column (Miltenyi Biotec, USA). A mixture was eluted from the column outside magnetic field in 400 µl of phosphate buffer, and fixed by adding 200 µl 4% para-formaldehyde solution. To control the sample volume, suspension of fixed EVs was supplemented with 50 µl counting beads of known concentration (AccuCheck, Invitrogen, Life Technologies). On the basis of volumetric measurement, we were able to recalculate number of events to EVs concentration in plasma.
Flow cytometry. A suspension of extracellular vesicles was analyzed using an Aria II flow cytometer (BD Bioscience) at speed ≤150 events/s. For analysis, all events with high width of fluorescence signal from Alexa Fluor 488 fluorochrome were considered as aggregates of EVs and neglected in analysis. Individual events detected from EVs passing through the laser system were determined according to height and width of fluorescence signal.
Efficacy and specificity of isolated EVs. Less than 1% of the EVs were stained by isotype-match control antibodies compared to staining with specific antibodies. Earlier, we documented high efficacy for isolating EVs [27]. These results were confirmed in the current study. During the first isolation procedure, 97% of total vesicles bearing a marker of interest were captured; by repeatedly isolating EVs from a sample that underwent a first-wave isolation, less than 3% out of initial population was re-captured. In addition, during the current study it was again checked that >90% events determined by flow cytometry corresponded to individual vesicles. Such results are in agreement with our earlier published data [28].
Statistical analysis. Normality of the data was checked by applying Pearson’s normality test and the Shapiro–Wilk test. In case of normal distribution, parameters were compared by using t-test. The data from several similar groups were compared by applying the one-way ANOVA test.
Pairwise comparisons were done using the Tukey test. When a distribution of the data did not match normality criteria, a Mann–Whitney test for pairwise comparisons and nonparametric Kruskal–Wallis test for comparing several groups were used. Pairwise comparisons were assessed using the nonparametric Dunn’s test. The χ2 test and Fisher’s exact test were used to analyze frequency difference in two independent groups. For normal distribution, the data were presented as mean ± standard deviation (M ± SD), and for non-normal distribution as median value and interquartile range. Statistical analysis was performed using Prism5.0d (GraphPad Software, USA) and Statistica 8.0 software. The level of statistical significance was set at α = 0.05.
RESULTS
Characterization of patients’ groups. During the study, the composition of extracellular vesicles from 17 healthy volunteers and 13 patients with ACS admitted to the hospital was analyzed. In the control group, seven men and ten women were recruited, average age 55.9 ± 9.0 years. In the group of patients with acute ischemic heart disease, nine men and four women were recruited having average age 61.8 ± 8.5 years. Later, the group of patients with ACS was subdivided into patients with acute myocardial infarction (six persons: five men and one woman, average age 61.7 ± 7.9 years) and patients with unstable angina (seven persons: four men and three women, average age 61.9 ± 9.7 years). In terms of the main parameters, no significant differences were found between group of patients with ACS as well as the subgroup of patients having AMI and UA versus healthy volunteers.
Content of EVs in patients with acute forms of IHD. EVs were isolated from PPP using MNPs carrying antibodies against surface markers: CD31, CD41a, and CD63. Then, for detection, vesicles were stained with fluorescently labeled antibodies against CD31 and CD63 in the case of CD41a-bound vesicles, against CD41 and CD63 in the case of CD31-bound vesicles, and against CD31 and CD41a in the case of CD63-bound vesicles (Fig. 1). An individual EV was determined as an event positively gated on MNP binding marker and expressing of at least one of detection markers. The concentrations of individual EVs were calculated based on the concentration of standard counting particles.
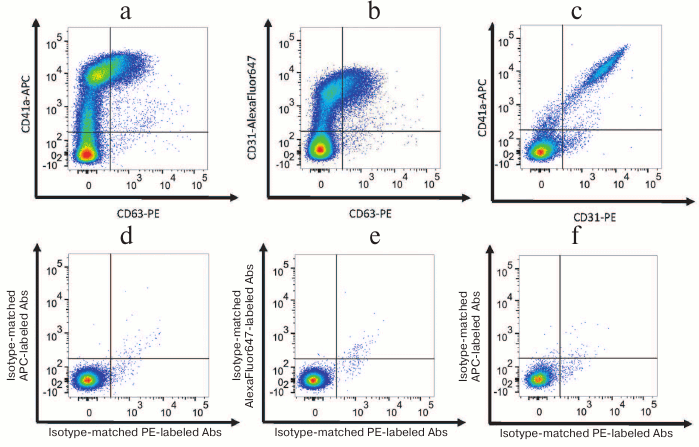
Fig. 1. EV–MNP complexes isolated from the blood of healthy volunteers (a representative flow cytometric analysis). a, d) EVs isolated using MNPs conjugated to antibodies against CD31 were stained with fluorescently labeled antibodies against CD63 and CD41a (a) or isotype-matched antibodies (d); b, e) EVs isolated by using MNPs conjugated to antibodies against CD41a were stained with fluorescently-labeled antibodies against CD63 and CD31 (b) or isotype-matched antibodies (e); c, f) EVs isolated by using MNPs conjugated to antibodies against CD63 were stained with fluorescently-labeled antibodies against CD31 and CD41a (c) or isotype-matched antibodies (f).
According to our data, the fraction of vesicles positive for platelet CD41a marker was the most abundant both in the blood of patients as well as healthy volunteers. In addition, the total number of vesicles isolated from the blood of patients and healthy volunteers using CD41a-MNPs that were positively stained for one or two antigens recognized by detection antibodies was significantly higher than the number of vesicles isolated by using CD31-MNPs and CD63-MNPs (3315 [2411; 6125] EVs/µl vs. 2213 [859; 3661] EVs/µl; p = 0.035; vs. 1908 [707; 3308] EVs/µl; p = 0.003, respectively).
During the study, we were able for the first time to determine that the total number of EVs isolated using either of the three test markers was substantially higher in patients with ACS compared to healthy volunteers. The number of EVs isolated by using CD31-MNPs that were positively stained by one or two detection antibodies was 3359 [2328; 5472] EVs/µl in patients with ACS compared to 1272 [714; 2157] EVs/µl in healthy volunteers (p = 0.001). The number of EVs isolated using CD63-MNPs and CD41a-MNPs was also higher in patients with ACS versus healthy volunteers (p = 0.001 and 0.015, respectively) (Table 1). For data with normal distribution, it was demonstrated that prevalence of EVs in patients with ACS versus control group was by 2.4-fold higher for those isolated with CD63-MNP vesicles (3207 ± 1827 vs. 1321 ± 1052; p = 0.002) and by 1.7-fold higher after using CD41a-MNP vesicles (5296 ± 2590 vs. 3069 ± 1555; p = 0.018).
Table 1. Total number of EVs isolated by
using MNP-immobilized antibodies from the blood of ACS patients versus
healthy volunteers
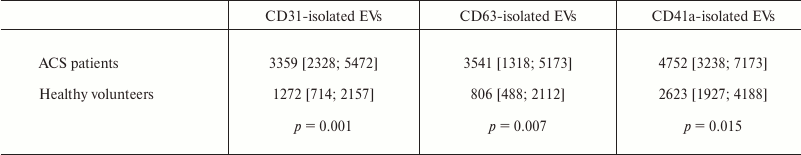
Note: EVs from ACS patients and healthy volunteers were isolated using
MNPs conjugated to one of the three antibodies against CD31, CD41a, or
CD63 and stained by the two other (fluorescent) antibodies,
respectively. Stained EV–MNP complexes were isolated on magnetic
columns for further analysis with flow cytometry. The data are
presented as median values, 25 and 75% percentiles of a number of EVs
per µl of blood plasma; p value was calculated by using
the Mann–Whitney test.
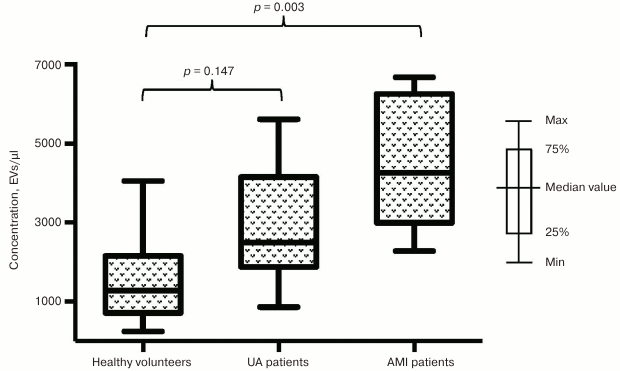
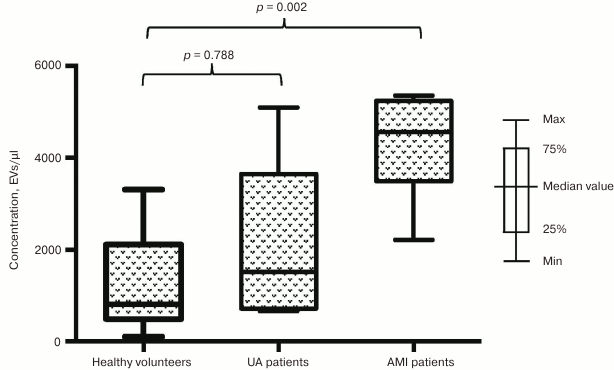
Next, we investigated the impact of a developing infarction zone on vesicle release and separately assessed distribution of EVs in subgroup of patients with AMI and UA. The prevalence of EVs in the subgroup of AMI patients was found to be even more significant compared to healthy volunteers for vesicle fractions isolated using both CD31-MNPs (4261 [2994; 6256] EVs/µl vs. 1272 [714; 2157] EVs/µl; p = 0.003) (Fig. 2) and CD63-MNPs (4559 [3489; 5238] EVs/µl vs. 806 [488; 2112] EVs/µl; p = 0.002) (Fig. 3). There was also a prevalence of EVs isolated using CD41a-MNPs found in AMI patients compared to healthy volunteers, but it did not reach a statistical significance. In contrast to the subgroup of AMI patients, it was found that the number of all analyzed types of EVs in UA patients did not differ significantly from that in healthy volunteers.
Fig. 2. EV–MNP complexes isolated from the blood of patients with UA and AMI using CD31 marker. EVs were isolated from patients with UA, AMI, and healthy volunteers using CD31-MNPs and stained with fluorescently labeled antibodies against CD41a and CD63. Stained EV–MNP complexes were isolated on magnetic columns and analyzed by using flow cytometry for counting. The p value was calculated using Dunn’s test.
Fig. 3. EV–MNP complexes isolated from the blood of patients with UA and AMI by using CD63. EVs from patients and healthy volunteers were isolated using CD63-MNPs and stained with fluorescently labeled antibodies against CD41a and CD31. Stained EV–MNP complexes were isolated on magnetic columns and analyzed using flow cytometry for counting. The p value was calculated using Dunn’s test.
Thus, we were able for the first time to demonstrate that by applying our original approach the number of individual extracellular vesicles in all tested fractions dominated in patients with acute forms of IHD compared to healthy volunteers, which was, in particular, evident in patients with developed myocardial infarction.
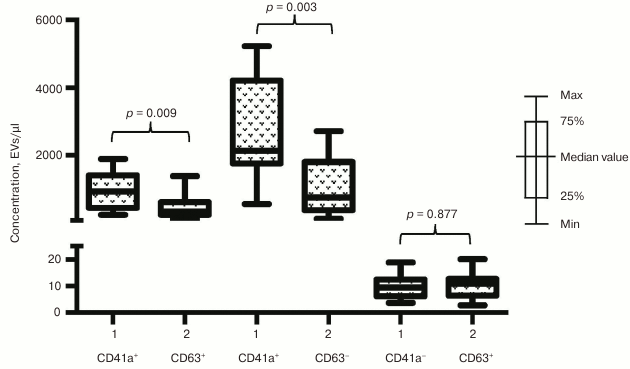
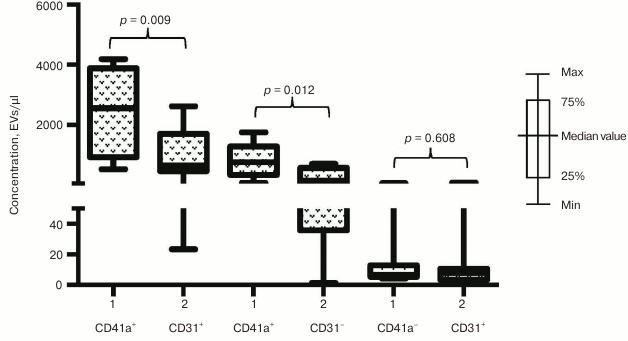
Phenotypic composition of EVs in ACS patients. We investigated whether the increase in EV numbers was a result of overall cell activation in response to developing ischemia or vesicles might be released selectively. To choose between these two possibilities we examined phenotypic composition of EVs in patients with acute forms of IHD compared to healthy volunteers. The number of vesicles isolated with CD31-MNPs and positive for CD41a and positive or negative for CD63 was significantly higher in ACS patients compared to healthy volunteers (940 [456; 1415] EVs/µl vs. 342 [246; 622] EVs/µl for CD63+; p = 0.009; and 2133 [1764; 4211] EVs/µl vs. 761 [385; 1807] EVs/µl for CD63−; p = 0.003, respectively). Interestingly, virtually no CD41a-negative EVs were found in this population of vesicles (Fig. 4). A similar distribution was detected by examining vesicles isolated with CD63-MNPs, which also virtually lacked CD41a-negative EVs, whereas vesicles positive for CD41a and positive or negative for CD31 were much more abundant in ACS patients compared to healthy volunteers (respectively, 2559 [931; 3885] EVs/µl vs. 659 [463; 1708] EVs/µl for CD31+; p = 0.009; and 758 [337; 1282] EVs/µl vs. 147 [36; 568] EVs/µl for CD31−; p = 0.012) (Fig. 5).
Fig. 4. EV–MNP complexes isolated from the blood of ACS patients and healthy volunteers using CD31 marker. EVs from ACS patients (1) and healthy volunteers (2) isolated using MNPs conjugated to antibodies against CD31 were stained with fluorescently labeled antibodies against CD41a and CD63. Stained EV–MNP complexes were isolated on magnetic columns and phenotyped by flow cytometer. The p value was calculated using the Mann–Whitney test.
Fig. 5. EV–MNP complexes isolated from the blood of ACS patients and healthy volunteers using CD63 marker. EVs from ACS patients (1) and healthy volunteers (2) isolated by using MNPs conjugated to antibodies against CD63 were stained with fluorescently labeled antibodies against CD41a and CD31. Stained EV–MNP complexes were isolated on magnetic columns and phenotyped by flow cytometer. The p value was calculated using the Mann–Whitney test.
Among EVs isolated using CD41a-MNPs, all subtypes of vesicles were more abundant in ACS patients compared to healthy volunteers. However, significant differences were found only in number of CD31+CD63− vesicles (4356 ± 2391 EVs/µl vs. 2199 ± 1112 EVs/µl; p = 0.010).
We decided to test if such distribution of EVs fractions was maintained in the larger pool of vesicles that was not limited by isolation according to the surface marker specific to certain cell types. For such isolation, we applied MNPs with ubiquitous MHC class I molecule [29], which can be considered as a unified marker for all types of vesicles [30, 31]. The vesicles were stained with antibodies against CD41a and CD31. Samples from patients and healthy volunteers were dominated by CD41a-positive CD31-positive vesicles. We showed that the number of EVs isolated from the blood of patients and healthy volunteers via MHC class I molecules being positive for both CD31 and CD41a did not significantly differ from number of EVs isolated using CD31 or CD41a and being positive for two other markers (2593 [1331; 4035] EVs/µl vs. 2213 [859; 3661] EVs/µl; p = 0.511; vs. 3315 [2411; 6125] EVs/µl; p = 0.109, respectively). The number of CD41a-positive vesicles isolated using MHC class I-MNPs was much higher in patients compared to healthy volunteers (2729 [1441; 3199] EVs/µl vs. 794 [522; 1415] EVs/µl; p = 0.032) (Table 2).
Table 2. MHC class I-isolated EVs in the
blood of ACS patients and healthy volunteers
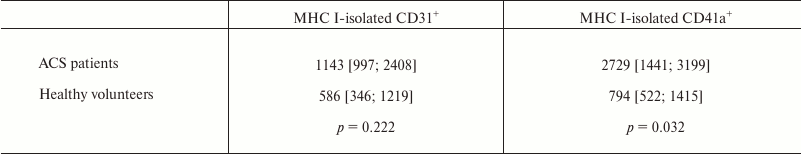
Note: EVs from ACS patients and healthy volunteers were isolated using
MNPs conjugated to antibodies against MHC class I molecules and stained
by fluorescent antibodies against CD41a and CD31. Stained EV–MNP
complexes were isolated on magnetic columns for further analysis with
flow cytometry. The data are presented as median values, 25 and 75%
percentiles of a number of EVs per µl of blood plasma. The
p value was calculated using the Mann–Whitney test.
Thus, we demonstrated that the abundance of EVs from the blood of ACS patients differed in various subsets of vesicles, being most significantly evident among subsets bearing platelet surface marker CD41a.
DISCUSSION
Release of vesicles by cells is a normal physiological process [32], which can alter during pathology [3, 4]. Due to this, EVs represent important candidates for playing a physiological role and serve as biomarkers in a number of diseases. It was demonstrated that the number of microparticles (fraction of EVs with large size) originating from endothelial cells, platelets, and monocytes as well as total number of microparticles increase in patients with acute and chronic forms of IHD [17, 18, 20, 21, 33-35], ischemic stroke [16, 17], endothelial dysfunction [36, 37], and people with high risk of cardiovascular diseases [15].
Evaluation of the individual phenotype of vesicles is considered as an important parameter characterizing physiological and pathological state of the cells that released them, based on the cell antigens detected on the surface of EVs. However, the majority of routine methods used to examine EVs analyze their phenotypic composition without determining phenotypic characteristics of individual vesicles. Here, we for the first time analyzed antigen composition of individual EVs in patients with acute coronary syndrome by adjusting our earlier proposed flow cytometry technique [27, 28].
Human EVs were bound to magnetic nanoparticles conjugated with antibodies recognizing various antigens on the surface of vesicles. Then the complexes were stained with other fluorescently labeled antibodies specific to the markers of interest. After magnetic separation of the bound vesicles from free antibodies, we were able to make use of fluorescence intensity rather than size of particles as a trigger analyzed during flow cytometry. It allowed us to detect <300 nm EVs, which were previously not possible to be examined by flow cytometry, which relies on the size of particles for triggering detection signal.
According to current understanding, there is no marker that can be associated only with one cell type. For binding of EVs and their subsequent staining, we used antibodies against three cell markers – CD31, CD41a, and CD63. The reason for choosing such markers was the previously demonstrated linkage between them and certain cell types as well as finding that the composition of large vesicles bearing such markers is changed during cardiovascular diseases. By combining antibodies recognizing these markers, this allowed us to detect subsets of EVs derived from platelets or endothelium. In addition, we also applied antibodies against MHC class I molecules, which are expressed on the majority of cell types [29]. We were interested in using CD31 and CD41a markers because it characterizes extracellular vesicles of both platelet (CD41a+CD31+/−) and endothelial (CD41a−CD31+) origin. CD63 is a tetraspanin, which in numerous studies was shown to play a role in formation of various EVs including endothelial EVs [12, 38]. Moreover, it was demonstrated that CD63 (traditionally considered to be a sign of activated platelets [39]) should be detected on the majority of platelet-derived vesicles, which can be released only upon activation or death of platelets due to structural features of the platelet membrane [40, 41]. Thus, use of this marker allowed additional characterization of EVs released both from platelets and from endothelium cells. Molecules of the major histocompatibility complex are considered as one of the most ubiquitous markers [29], which we also used to conduct a broader analysis of various types of extracellular vesicles and to test the three markers mentioned above, allowing us to isolate and characterize virtually all blood EVs bearing platelet and endothelial markers. We found that the number of EVs isolated by using MHC class I molecules, which were collectively positive for CD31 and CD41a expression, did not differ significantly from the number of EVs isolated by using CD31 or CD41a that were positive for expression of two other markers. Thus, this proved that the analysis was related not to a subset of platelet- and endothelium-derived vesicles, but to all of them.
Using our original approach, we were able to count the number of various subsets of individual EVs and to compare them between healthy volunteers and ACS patients.
Isolation of EVs on magnetic columns was found to be highly efficient: we were able to bind ~97% of the EVs, i.e. we were able to isolate virtually all EVs bearing a test antigen on their surface. We compared total number of EVs isolated by using antibodies against the three above-mentioned markers in ACS patients and healthy volunteers. In all cases, we observed a substantial prevalence of EVs in the blood plasma from ACS patients compared to the healthy volunteers. These results were also confirmed for EVs isolated using antibodies against MHC class I molecules. Then we investigated whether increased number of EVs correlated with the development of acute myocardial infarction. We found that in terms of the number of EVs, the greatest difference was observed between control group and subgroup of AMI patients. Our data are in agreement with the results previously published upon the analysis of a separate fraction of microparticles [33].
The technique developed by us allowed switching from analysis of overall pool of vesicles to individual evaluation of EVs including the smallest ones (which, according to Pol et al. [23], comprise the majority of EVs in blood). We found that the most abundant in plasma were EVs isolated using different MNPs bearing surface marker CD41a, suggesting their platelet-derived origin. Such data are in agreement with the results of numerous studies examining the overall pool of EVs in ACS patients [34, 35, 42]. However, they differ from the results obtained by Biasucci et al. [33], who found a significant number of CD31+CD41a− EVs in plasma. Such a discrepancy may appear because in our study we analyzed mainly small EVs, whereas in the latter study larger EVs were examined (microparticles and apoptotic bodies), which might be analyzed by applying a standard flow cytometry unable to detect particles with the size less than 300 nm.
To summarize, we found that the dominant subset of EVs isolated from the blood of ACS patients and healthy volunteers expressed CD41a on their surface, thus suggesting their platelet-derived origin. The total number of EVs isolated using antibodies against examined markers was higher in ACS patients compared to healthy volunteers. It was noted that the blood EVs were mostly prevalent in the subgroup of AMI patients compared to the control group. Note that increased number of EVs in ACS patients was not observed in all subsets of EVs, but mainly was due to the increase in CD41a+ EVs. The increased number of platelet-derived EVs in the blood of ACS patients probably reflects platelet activation during acute myocardial ischemia [43, 44].
The composition and number of EVs are important parameters of general biochemical profile of blood. Further investigations will reveal whether an increased number of EVs might be used as one of the earliest biomarkers of developing ACS. Altogether, analysis of antigenic composition of individual extracellular vesicles determining their cell origin opens up an opportunity to obtain large-scale data regarding the state of body cells that released them, as well as signs of disease progression.
Research by A. Arakelyan, J.-Ch. Grivel, and L. Margolis was performed within a framework of an intramural program of the National Institute of Child Health and Human Development, NIH.
This study was financially supported by the Government of the Russian Federation (project No. 14.B25.31.0016).
REFERENCES
1.Ong, S.-G., Lee, W. H., Huang, M., Dey, D., Kodo,
K., Sanchez-Freire, V., Gold, J. D., and Wu, J. C. (2014) Cross talk of
combined gene and cell therapy in ischemic heart disease: role of
exosomal microRNA transfer, Circulation, 130,
S60-S69.
2.Zhang, J., Li, S., Li, L., Li, M., Guo, C., Yao,
J., and Mi, S. (2015) Exosome and exosomal microRNA: trafficking,
sorting, and function, Genom. Proteom. Bioinform., 13,
17-24.
3.Yuana, Y., Sturk, A., and Nieuwland, R. (2013)
Extracellular vesicles in physiological and pathological conditions,
Blood Rev., 27, 31-39.
4.Record, M., Carayon, K., Poirot, M., and
Silvente-Poirot, S. (2014) Exosomes as new vesicular lipid transporters
involved in cell–cell communication and various
pathophysiologies, Biochim. Biophys. Acta, 1841,
108-120.
5.Rautou, P. E., Vion, A. C., Amabile, N., Chironi,
G., Simon, A., Tedgui, A., and Boulanger, C. M. (2011) Microparticles,
vascular function, and atherothrombosis, Circ. Res., 109,
593-606.
6.Ayers, L., Harrison, P., Kohler, M., and Ferry, B.
(2014) Procoagulant and platelet-derived microvesicle absolute counts
determined by flow cytometry correlates with a measurement of their
functional capacity, J. Extracell. Vesicles, doi:
10.3402/jev.v3.25348.
7.Lacroix, R., Dubois, C., Leroyer, A. S., Sabatier,
F., and Dignat-George, F. (2013) Revisited role of microparticles in
arterial and venous thrombosis, J. Thromb. Haemost., 11
(Suppl. 1), 24-35.
8.Leroyer, A. S., Tedgui, A., and Boulanger, C. M.
(2008) Role of microparticles in atherothrombosis, J. Intern.
Med., 263, 528-537.
9.Nomura, S., and Shimizu, M. (2015) Clinical
significance of procoagulant microparticles, J. Intens. Care,
3, 2.
10.De Toro, J., Herschlik, L., Waldner, C., and
Mongini, C. (2015) Emerging roles of exosomes in normal and
pathological conditions: new insights for diagnosis and therapeutic
applications, Front. Immunol., 6, 203.
11.Zocco, D., Ferruzzi, P., Cappello, F., Kuo, W.
P., and Fais, S. (2014) Extracellular vesicles as shuttles of tumor
biomarkers and anti-tumor drugs, Front. Oncol., 4,
267.
12.Colombo, M., Moita, C., Van Niel, G., Kowal, J.,
Vigneron, J., Benaroch, P., Manel, N., Moita, L. F., Thery, C., and
Raposo, G. (2013) Analysis of ESCRT functions in exosome biogenesis,
composition and secretion highlights the heterogeneity of extracellular
vesicles, J. Cell Sci., 126, 5553-5565.
13.Besancenot, R., Chaligne, R., Tonetti, C.,
Pasquier, F., Marty, C., Lecluse, Y., Vainchenker, W., Constantinescu,
S. N., and Giraudier, S. (2010) A senescence-like cell-cycle arrest
occurs during megakaryocytic maturation: implications for physiological
and pathological megakaryocytic proliferation, PLoS Biol.,
8.
14.Aatonen, M. T., Ohman, T., Nyman, T. A.,
Laitinen, S., Gronholm, M., and Siljander, P. R. (2014) Isolation and
characterization of platelet-derived extracellular vesicles, J.
Extracell. Vesicles, 3, 1-15.
15.Amabile, N., Cheng, S., Renard, J. M., Larson, M.
G., Ghorbani, A., McCabe, E., Griffin, G., Guerin, C., Ho, J. E., Shaw,
S. Y., Cohen, K. S., Vasan, R. S., Tedgui, A., Boulanger, C. M., and
Wang, T. J. (2014) Association of circulating endothelial
microparticles with cardiometabolic risk factors in the Framingham
Heart Study, Eur. Heart J., 1-8.
16.Li, P., and Qin, C. (2015) Elevated circulating
VE-cadherin+ CD144+ endothelial microparticles in
ischemic cerebrovascular disease, Thromb. Res., 135,
375-381.
17.Hu, S.-S., Zhang, H.-G., Zhang, Q.-J., and Xiu,
R.-J. (2014) Small-size circulating endothelial microparticles in
coronary artery disease, PLoS One, 9, e104528.
18.Jung, C., Sorensson, P., Saleh, N., Arheden, H.,
Ryden, L., and Pernow, J. (2012) Circulating endothelial and platelet
derived microparticles reflect the size of myocardium at risk in
patients with ST-elevation myocardial infarction,
Atherosclerosis, 221, 226-231.
19.Gyorgy, B., Szabo, T. G., Pasztoi, M., Pal, Z.,
Misjak, P., Aradi, B., Laszlo, V., Pallinger, E., Pap, E., Kittel, A.,
Nagy, G., Falus, A., and Buzas, E. I. (2011) Membrane vesicles, current
state-of-the-art: emerging role of extracellular vesicles, Cell.
Mol. Life Sci., 68, 2667-2688.
20.Stepien, E., Stankiewicz, E., Zalewski, J.,
Godlewski, J., Zmudka, K., and Wybranska, I. (2012) Number of
microparticles generated during acute myocardial infarction and stable
angina correlates with platelet activation, Arch. Med. Res.,
43, 31-35.
21.Skeppholm, M., Mobarrez, F., Malmqvist, K., and
Wallen, H. (2012) Platelet-derived microparticles during and after
acute coronary syndrome, Thromb. Haemost., 107,
1122-1129.
22.Thygesen, K., Alpert, J. S., Jaffe, A. S.,
Simoons, M. L., Chaitman, B. R., White, H. D., Joint ESC/ACCF/AHA/WHF
task force for universal definition of myocardial infarction (2012)
Third universal definition of myocardial infarction, J. Am. Coll.
Cardiol., 60, 1581-1598.
23.Van der Pol, E., Coumans, F. A. W., Grootemaat,
A. E., Gardiner, C., Sargent, I. L., Harrison, P., Sturk, A., Van
Leeuwen, T. G., and Nieuwland, R. (2014) Particle size distribution of
exosomes and microvesicles determined by transmission electron
microscopy, flow cytometry, nanoparticle tracking analysis, and
resistive pulse sensing, J. Thromb. Haemost., 12,
1182-1192.
24.Witwer, K. W., Buzas, E. I., Bemis, L. T., Bora,
A., Lasser, C., Lotvall, J., Nolte-’t Hoen, E. N., Piper, M. G.,
Sivaraman, S., Skog, J., Thery, C., Wauben, M. H., and Hochberg, F.
(2013) Standardization of sample collection, isolation and analysis
methods in extracellular vesicle research, J. Extracell.
Vesicles, 2.
25.Task Force on the management of ST-segment
elevation acute myocardial infarction of the European Society of
Cardiology (ESC), Steg, P. G., James, S. K., Atar, D., Badano, L. P.,
Blomstrom-Lundqvist, C., Borger, M. A., Di Mario, C., Dickstein, K.,
Ducrocq, G., Fernandez-Aviles, F., Gershlick, A. H., Giannuzzi, P.,
Halvorsen, S., Huber, K., Juni, P., Kastrati, A., Knuuti, J., Lenzen,
M. J., Mahaffey, K. W., Valgimigli, M., Van’t Hof, A., Widimsky,
P., and Zahger, D. (2012) ESC guidelines for the management of acute
myocardial infarction in patients presenting with ST-segment elevation,
Eur. Heart J., 33, 2569-2619.
26.Hamm, C. W., Bassand, J.-P., Agewall, S., Bax,
J., Boersma, E., Bueno, H., Caso, P., Dudek, D., Gielen, S., Huber, K.,
Ohman, M., Petrie, M. C., Sonntag, F., Uva, M. S., Storey, R. F.,
Wijns, W., and Zahger, D. (2011) ESC guidelines for the management of
acute coronary syndromes in patients presenting without persistent
ST-segment elevation: the task force for the management of acute
coronary syndromes (ACS) in patients presenting without persistent
ST-segment elevatio, Eur. Heart J., 32, 2999-3054.
27.Arakelyan, A., Ivanova, O., Vasilieva, E.,
Grivel, J.-C., and Margolis, L. (2015) Antigenic composition of single
nano-sized extracellular blood vesicles, Nanomedicine,
11, 489-498.
28.Arakelyan, A., Fitzgerald, W., Margolis, L., and
Grivel, J. (2013) Nanoparticle-based flow virometry for the analysis of
individual virions, J. Clin. Invest., 123, 3716-3727.
29.Campbell, E. C., Antoniou, A. N., and Powis, S.
J. (2012) The multi-faceted nature of HLA class I dimer molecules,
Immunology, 136, 380-384.
30.Andre, F., Chaput, N., Schartz, N. E. C.,
Flament, C., Aubert, N., Bernard, J., Lemonnier, F., Raposo, G.,
Escudier, B., Hsu, D.-H., Tursz, T., Amigorena, S., Angevin, E., and
Zitvogel, L. (2004) Exosomes as potent cell-free peptide-based vaccine.
I. Dendritic cell-derived exosomes transfer functional MHC class
I/peptide complexes to dendritic cells, J. Immunol., 172,
2126-2136.
31.Lynch, S., Santos, S. G., Campbell, E. C., Nimmo,
A. M. S., Botting, C., Prescott, A., Antoniou, A. N., and Powis, S. J.
(2009) Novel MHC class I structures on exosomes, J. Immunol.,
183, 1884-1891.
32.Thery, C. (2011) Exosomes: secreted vesicles and
intercellular communications, F1000 Biol. Rep., 3,
15.
33.Biasucci, L. M., Porto, I., Di Vito, L., De
Maria, G. L., Leone, A. M., Tinelli, G., Tritarelli, A., Di Rocco, G.,
Snider, F., Capogrossi, M. C., and Crea, F. (2012) Differences in
microparticle release in patients with acute coronary syndrome and
stable angina, Circ. J., 76, 2174-2182.
34.Kafian, S., Mobarrez, F., Wallen, H., and Samad,
B. (2014) Association between platelet reactivity and circulating
platelet-derived microvesicles in patients with acute coronary
syndrome, Platelets, 7104, 1-7.
35.Morel, O., Pereira, B., Averous, G., Faure, A.,
Jesel, L., Germain, P., Grunebaum, L., Ohlmann, P., Freyssinet, J. M.,
Bareiss, P., and Toti, F. (2009) Increased levels of procoagulant
tissue factor-bearing microparticles within the occluded coronary
artery of patients with ST-segment elevation myocardial infarction:
role of endothelial damage and leukocyte activation,
Atherosclerosis, 204, 636-641.
36.Lovren, F., and Verma, S. (2013) Evolving role of
microparticles in the pathophysiology of endothelial dysfunction,
Clin. Chem., 59, 1166-1174.
37.Helbing, T. (2014) Role of microparticles in
endothelial dysfunction and arterial hypertension, World J.
Cardiol., 6, 1135.
38.Bobrie, A., Colombo, M., Krumeich, S., Raposo,
G., and Thery, C. (2012) Diverse subpopulations of vesicles secreted by
different intracellular mechanisms are present in exosome preparations
obtained by differential ultracentrifugation, J. Extracell.
Vesicles, 1, 1-11.
39.Metzelaar, M. J., Wijngaard, P. L. J., Peters, P.
J., Sixma, J. J., Nieuwenhuis, H. K., and Clevers, H. C. (1991) CD63
antigen: a novel lysosomal membrane glycoprotein, cloned by a screening
procedure for intracellular antigens in eukaryotic cells, J. Biol.
Chem., 266, 3239-3245.
40.Piccin, A., Murphy, W. G., and Smith, O. P.
(2007) Circulating microparticles: pathophysiology and clinical
implications, Blood Rev., 21, 157-171.
41.Antwi-Baffour, S., Adjei, J., Aryeh, C.,
Kyeremeh, R., Kyei, F., and Seidu, M. A. (2015) Understanding the
biosynthesis of platelets-derived extracellular vesicles, Immun.
Inflamm. Dis., 3, 133-140.
42.Min, P.-K., Kim, J.-Y., Chung, K.-H., Lee, B. K.,
Cho, M., Lee, D.-L., Hong, S.-Y., Choi, E.-Y., Yoon, Y.-W., Hong,
B.-K., Rim, S.-J., and Kwon, H. M. (2013) Local increase in
microparticles from the aspirate of culprit coronary arteries in
patients with ST-segment elevation myocardial infarction,
Atherosclerosis, 227, 323-328.
43.Patrono, C., and Renda, G. (1997) Platelet
activation and inhibition in unstable coronary syndromes, Am. J.
Cardiol., 80, 17-20.
44.Harker, L. A., and Ritchie, J. L. (1980) The role
of platelets in acute vascular events, Circulation, 62,
13-18.