Photosystem II Activity of Wild Type Synechocystis PCC 6803 and Its Mutants with Different Plastoquinone Pool Redox States
O. V. Voloshina1*, Y. V. Bolychevtseva2*, F. I. Kuzminov1,3, M. Y. Gorbunov3, I. V. Elanskaya4, and V. V. Fadeev1
1Lomonosov Moscow State University, International Laser Center, 119991 Moscow, Russia; E-mail: olgavproskurina@gmail.com2Bach Institute of Biochemistry, Research Center of Biotechnology, Russian Academy of Sciences, 119071 Moscow, Russia; E-mail: bolychev@inbi.ras.ru, bolychev1@yandex.ru
3Rutgers, The State University of New Jersey, Department of Marine and Coastal Sciences, 08901 New Brunswick, New Jersey, USA
4Lomonosov Moscow State University, Faculty of Biology, 119991 Moscow, Russia
* To whom correspondence should be addressed.
Received December 21, 2015; Revision received April 28, 2016
To assess the role of redox state of photosystem II (PSII) acceptor side electron carriers in PSII photochemical activity, we studied sub-millisecond fluorescence kinetics of the wild type Synechocystis PCC 6803 and its mutants with natural variability in the redox state of the plastoquinone (PQ) pool. In cyanobacteria, dark adaptation tends to reduce PQ pool and induce a shift of the cyanobacterial photosynthetic apparatus to State 2, whereas illumination oxidizes PQ pool, leading to State 1 (Mullineaux, C. W., and Holzwarth, A. R. (1990) FEBS Lett., 260, 245-248). We show here that dark-adapted Ox– mutant with naturally reduced PQ is characterized by slower QA– reoxidation and O2 evolution rates, as well as lower quantum yield of PSII primary photochemical reactions (Fv/Fm) as compared to the wild type and SDH– mutant, in which the PQ pool remains oxidized in the dark. These results indicate a large portion of photochemically inactive PSII reaction centers in the Ox– mutant after dark adaptation. While light adaptation increases Fv/Fm in all tested strains, indicating PSII activation, by far the greatest increase in Fv/Fm and O2 evolution rates is observed in the Ox– mutant. Continuous illumination of Ox– mutant cells with low-intensity blue light, that accelerates QA– reoxidation, also increases Fv/Fm and PSII functional absorption cross-section (590 nm); this effect is almost absent in the wild type and SDH– mutant. We believe that these changes are caused by the reorganization of the photosynthetic apparatus during transition from State 2 to State 1. We propose that two processes affect the PSII activity during changes of light conditions: 1) reversible inactivation of PSII, which is associated with the reduction of electron carriers on the PSII acceptor side in the dark, and 2) PSII activation under low light related to the increase in functional absorption cross-section at 590 nm.
KEY WORDS: cyanobacteria, mutants, photosystem II, plastoquinone pool, state transitions of photosynthetic apparatusDOI: 10.1134/S000629791608006X
Abbreviations: DBMIB, 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone (dibromo-thymoquinone); DCMU, 3-(3,4-dichlorophenyl)-1,1′-dimethylurea (diuron); Fd, ferredoxin; FNR, ferredoxin-NADP oxidoreductase; Ox–, a terminal oxidase-lacking mutant; PBS, phycobilisome; PQ, plastoquinone; PSII (PSI), photosystem 2 (1); QA and QB, primary and secondary PSII quinone electron acceptors; SDH–, a succinate dehydrogenase-lacking mutant.
Photosynthetic organisms are extremely sensitive to changes in light
conditions and are able to change pigment composition of the
light-harvesting complexes and the ratio of the main
pigment–protein complexes to achieve maximum photosynthetic
efficiency. This is reflected in the adaptive changes of the pigment
apparatus and the rates of photochemical processes. In particular, upon
changes in light conditions, cyanobacteria, higher plants, and algae
are able to redistribute absorbed light energy between PSI and PSII,
allowing them to balance electron transport. This is achieved through
the self-regulating system with two stable states (State 1 and State
2), known as short-term changes in photosynthetic apparatus
organization [1-5]. Change in
the pigment apparatus state depend on the redox state of electron
carriers that transfer electrons between the photosystems [5-7]. Under light 1, absorbed
predominantly by PSI, the balance of linear electron transport is
shifted. This causes oxidation of the PQ pool and cytochrome
b6/f, and the photosynthetic apparatus
(seeking to restore the balance) is transformed to State 1, which is
characterized by an increase in the portion of energy absorbed by PSII
(this process is associated with migration of the light-harvesting
complexes from PSI to PSII). The transition to State 2, accompanied by
an increase in the portion of light absorbed by PSI, is caused by an
accumulation of reduced electron carriers between the photosystems due
to excessive donation of electrons by PSII caused by light 2, absorbed
predominantly by this photosystem [4-8].
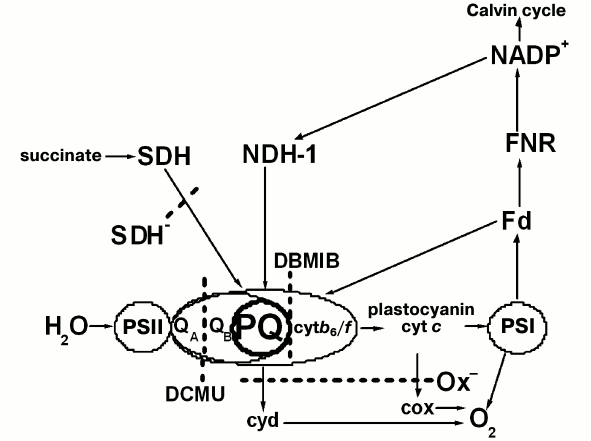
The regulatory mechanism of the transition between stable states in cyanobacteria differs from that in plants due to differences in the organization of the light-harvesting apparatus. Unlike the plant thylakoid membrane, cyanobacterial thylakoid membranes lack the integral chlorophyll a/b-light-harvesting complex. The main light-harvesting complexes of cyanobacteria are phycobiliprotein complexes, phycobilisomes (PBS), located on the outside of the thylakoid membrane, that effectively transfer absorbed light energy to PSII and PSI [9]. Despite the differences in thylakoid membrane organization, all photosynthetic organisms are capable of transitions of their photosynthetic apparatus between States 1 and 2 in response to different redox state of the PQ pool and the cytochrome b6/f complex [5-7]. In contrast to eukaryotic organisms, cyanobacteria can transfer to State 2 even in the dark. This is possible because the cyanobacterial thylakoid membranes contain both photosynthetic electron carriers (e.g. PQ, cytochrome b6/f, PSI, PSII) and the respiratory chain carriers like NDH-1 and succinate dehydrogenase (SDH) that can reduce the PQ pool [10-14], and terminal oxidases [15], which facilitate PQ pool oxidation at abundance of a reducing agent (Fig. 1).
Fig. 1. Possible electron transfer pathways in thylakoid membranes of wild type and mutant Synechocystis PCC 6803 cells: the mutant lacking terminal oxidases (Ox–) and the one lacking succinate dehydrogenase (SDH–). Cyt b6/f, cytochrome b6/f complex; cyt c, cytochrome c; cyd and cox, terminal plastoquinol oxidase and cytochrome c oxidase, respectively; NDH-1, type 1 NAD(P)H dehydrogenase; SDH, succinate dehydrogenase. The remaining designations are given in the abbreviations list. The dotted line indicates active sites of PSII inhibitors (DCMU and DBMIB) cutting off electron transfer pathways in mutants.
In contrast to State 2, State 1 of the cyanobacterial photosynthetic apparatus, caused by the oxidized PQ pool, is characterized by a high PSII fluorescence yield in the presence of DCMU at room temperature and a high PSII relative fluorescence yield (at wavelengths of 685 and 692 nm) in 77K fluorescence spectra [1, 7, 8, 11, 16, 17].
To explain the nature of processes underlying the redistribution of the energy absorbed by PBS between the photosystems, several mechanisms have been proposed: a change in the effective absorption cross-section of PSII and PSI caused by PBS migration between photosystems [1, 18]; a change in the degree of complex aggregation of photosystems: trimer–monomer for PSI and dimer–monomer for PSII [16, 19-21]; mobility of all pigment–protein complexes, and energy transfer by a “spillover” mechanism [22]. However, the mechanism by which the oxidation state of electron carriers between the photosystems control energy migration processes in cyanobacteria is not yet established [17].
Previously, it was shown that in the dark cyanobacteria transfer electrons from stroma to PQ pool by reducing plastoquinones with succinate dehydrogenase; therefore, a mutant lacking succinate dehydrogenase has a PQ pool that is more oxidized in the dark than that of the wild type [23]. In Synechocystis Ox– mutant cells lacking terminal oxidases (and, therefore, a PQ pool oxidation pathway), the PQ pool is reduced compared to the wild type and SDH– mutant [24]. It was assumed earlier that a changes in photosynthetic apparatus organization during transition between States 1 and 2 in cyanobacteria might be controlled not only by the redox state of the PQ pool, but also by the rate of cyclic electron transport through PSI and a transmembrane electrochemical potential formed on the thylakoid membrane [4, 6]. Additionally, a dependence of PBS mobility on the redox state of the primary PSII quinone acceptor (QA) was found [25]. As the mechanism of redox signal transfer from inter-photosystem electron carriers to structures capable of affecting energy migration processes in cyanobacteria is not sufficiently clear, it was interesting to understand how the redox state of the PSII quinone acceptor (QA) can affect PSII activity under different states of the photosynthetic apparatus.
The goal of the current study was to investigate the relationship between changes in redox state of the quinone component (QA) of the PSII acceptor site, located on the D2 protein of the PSII core complex [26], and PSII activity during photosynthetic apparatus reorganization caused by transition between States 1 and 2 under different light conditions. Our approach to this problem was to use Synechocystis cyanobacteria mutants with different redox states of the PQ pool.
Our study has shown dark-adapted Ox– mutant cells with reduced PQ pool to be characterized by slower QA– reoxidation, lower probability of energy transfer between PSII reaction centers (ρ), and lower quantum yield of the primary PSII photochemical reactions as compared to the wild type and SDH– mutant cells with oxidized PQ pool. These results are also confirmed by a lower oxygen evolution rate for Ox– cells, indicating a larger portion of photochemically inactive (i.e. not possessing variable fluorescence) PSII reaction centers in dark-adapted Ox– cells as compared to the wild type and SDH– mutant cells. Low continuous light that “switched on” linear electron transfer (acceleration of QA– reoxidation) was also found to increase the functional absorption cross-section for 590 nm light for PSII (σ590) and the quantum yield for the primary photochemical reaction (Fv/Fm) in the Ox– mutant, but not to alter Fv/Fm and the functional absorption cross-section of PSII (σ450). Such changes in PSII fluorescence reflect changes in the cyanobacterial photosynthetic apparatus during the transition from State 2 to State 1.
MATERIALS AND METHODS
Cyanobacterial strains and cultivation conditions. For this study, Synechocystis PCC 6803 cyanobacteria of wild type and Ox– (ctaCDE–/cydAB–/ctaDEII–, lacking terminal oxidases) [27] and SDH– (sll1625–/sll0823–, lacking succinate dehydrogenase) [14] mutants were used. The Synechocystis strain cells were grown photoautotrophically for 4-5 days at 30°C under continuous illumination with daylight (40 µmol photons·m–2·s–1) and continuous stirring in liquid mineral BG11 medium [28] with the addition of appropriate antibiotics.
Sample preparation. Cells were collected by filtration on filters (Nalgene; pore size 0.45 µm, volume 150 ml) with a hand-operated vacuum pump (Nalgene) and diluted by the cultivation medium to the desired suspension density. Suspension density was characterized by chlorophyll concentration. Chlorophyll concentration was measured in 80% acetone extracts according to the Lichtenthaler method [29]. The optical density of extracts was determined with a Beckman DU-650 spectrophotometer (Beckman Coulter Inc., USA) in a standard 1-cm pathlength cuvette. Cell absorption spectra were measured at room temperature with a Hitachi-557 spectrophotometer (Japan) in a standard 1-cm cuvette.
Dark and light adaptation of cells. For dark adaptation, collected cells were incubated in the dark for no less than 30 min; for light adaptation, cells were illuminated for 20 min with white light from an incandescent lamp (25 µmol photons·m–2·s–1). Light adaptation was always carried out on cells that were preliminarily incubated in the dark for 30 min.
PSII fluorescence induction in Synechocystis cells was registered in pulsed illumination mode in the presence of 5·10–5 M DCMU with a PAM-101 fluorometer [30]. The source of actinic light of 620 nm (40 µmol photons·m–2·s–1) was a controlled LED-based lamp (High Power Led Lamp, control unit HPL-C; Walz, Germany); the flash time was 1 s, and the interval between flashes was 15 s. Measurements were carried out at sample chlorophyll concentration of 10 µg/ml in a 1-cm cuvette. The intensity of actinic 620 nm light was measured with a Quantitherm quantometer (QRT1; Hansatech, Germany).
Plastoquinone pool redox state was evaluated according to the Berry method [31]. The cell suspension was preliminarily normalized to chlorophyll concentration of 10 µg/ml. DCMU was added to an aliquot of cell suspension to final concentration 5·10–5 M; dibromothymoquinone (DBMIB) concentration of 60 µM and ascorbate (5 mM) were added to another aliquot. Fluorescence induction kinetics of the samples were registered with the PAM-101 device with a source of 620 nm actinic light (40 µmol photons·m–2·s–1).
PSII oxygen evolution was measured for cell suspension in culture medium (chlorophyll concentration 20 µg/ml) by a potentiometer with a Clark electrode (Oxygraph; Hansatech) in a thermostatted 1 ml cuvette at 28°C. Photochemical activity of PSII was measured with the natural donor (H2O) and acceptor (CO2) and evaluated in µmol O2/mg Chl per hour. An OI-24 illuminator (LOMO, Russia) was used as the white light source, and the illumination intensity was 800 µmol photons·m–2·s–1.
Fluorescence induction and relaxation kinetics. The kinetics of induction and relaxation of the PSII fluorescence was measured using a custom build Fluorescence Induction and Relaxation (FIRe)-System [32]. Fluorescence was excited by blue (450 nm with 30 nm half bandwidth) and orange (590 nm with 30 nm bandwidth) light-emitting diodes and recorded in the red spectral region (680 nm with 20 nm bandwidth). The diodes generate single saturating pulses with the peak optical power density up to 1 W/cm2 (0.1 ms for 590-nm light and 0.3 ms for 450-nm light) to ensure fast reduction of PSII reaction centers within a single photosynthetic turnover (~100 μs). Kinetics were measured in two modes – under only pulse light and under additional continuous LED light (470 nm, 20 µmol photons·m–2·s–1). For calculations, kinetics were averaged over 50 saturating pulses, the time between pulses being 0.5 s.
Analysis of the fluorescence induction kinetics allows calculation of mean values of initial fluorescence (F0), maximum fluorescence (Fm), quantum yield of the primary photochemical reaction (Fv/Fm), functional absorption cross-section of PSII (σPSII) [33], and the ρ (or connectivity) factor, which is indicative of the possibility for energy exchange between photosynthetic units [34, 35]. Analysis of fluorescence relaxation kinetics allows calculating τQA value – the average time of QA– reoxidation.
RESULTS AND DISCUSSION
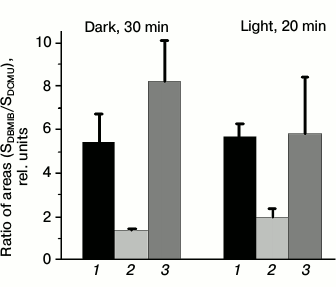
Evaluating plastoquinone pool redox state of wild type and mutant Synechocystis cells with the PAM-101. The photosynthetic apparatus state is known to depend on the redox state of the electron carriers that transfer electrons between the photosystems [5-7]. To test that, we measured fluorescence induction with the PAM-101 to estimate the PQ pool redox state in wild type and mutant Synechocystis cells using method described by Berry and co-authors [31]. The method is based on comparing areas above the fluorescence induction curve in the presence of DBMIB and ascorbate (SDBMIB) and fluorescence induction curve in the presence of DCMU (SDCMU). The ratio of these areas (SDBMIB/SDCMU) was used as an indicator of PQ pool redox state. For dark- or light-adapted Ox– mutant cells, this value was the lowest (Fig. 2) as compared to wild type and SDH– cells, which is in agreement with data on the highest pool reduction level for the Ox– mutant lacking terminal oxidases [24]. From the SDBMIB/SDCMU ratio for dark- or light-adapted wild type cells, the PQ pool redox state was almost the same under the applied illumination conditions (Fig. 2). A higher SDBMIB/SDCMU value of dark-adapted SDH– cells, compared to that of wild type cells incubated in the dark, indicates a more oxidized PQ pool for the SDH– mutant, which is explained by the absence of succinate dehydrogenase in the thylakoid membranes [23]. A decrease in SDBMIB/SDCMU value of light-adapted SDH– cells compared to that of SDH– cells incubated in the dark (Fig. 2) indicates reduction of the PQ pool in SDH– cells during light adaptation.
Fig. 2. Ratio of areas (SDBMIB/SDCMU) above the curves of variable fluorescence, calculated for wild type Synechocystis cells (1) and Ox– (2) and SDH– mutants (3), incubated for 30 min in the dark or illuminated for 20 min with white light, 25 µmol photons·m–2·s–1. SDBMIB is the area above the variable fluorescence curve in the presence of 60 µM DBMIB and 0.5 mM ascorbate; SDCMU is the area above the variable fluorescence curve in the presence of 5·10–5 M DCMU; the ratios are mean values ± SD taken from the values obtained in three independent experiments.
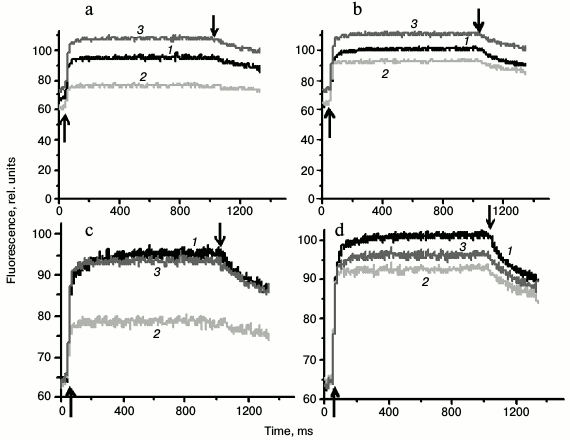
PSII fluorescence induction kinetics in Synechocystis cells measured using PAM-101. To estimate cyanobacterial photosynthetic apparatus states, the we used (i) value of PSII fluorescence quantum yield at room temperature and (ii) the intensity of PSII fluorescence (at 685 and 692 nm) relative to that of PSI (at 720 nm) in the low-temperature (77 K) fluorescence spectra [1, 7, 8, 10, 11, 16, 17]. The fluorescence of Synechocystis cultures used in the current study was earlier analyzed by pulse fluorometry (PAM-101) and low-temperature (77 K) fluorometry with a Shimadzu RF-5301PC spectrofluorometer (Japan) [36]. Repeated measurements with the PAM-101 fluorometer for cells adapted to light conditions described in this study confirmed results obtained earlier. The Ox– mutant cells, incubated for 30 min in the dark, were characterized by 1.5-1.7 times lower variable fluorescence yield in the presence of DCMU as compared to the wild type and SDH– mutant cells (Fig. 3, a and c). However, after 20 min of incubation under white light, the fluorescence of Ox– cells increases noticeably, while the fluorescence of wild type and SDH– cells changes insignificantly (Fig. 3, b and d).
Fig. 3. Typical fluorescence induction kinetics, measured at room temperature for wild type Synechocystis cells (1) and Ox– (2) and SDH– mutants (3) incubated for 30 min in the dark (a and c) or illuminated for 20 min by white light, 25 µmol photons·m–2·s–1 (b and d). The same kinetics, normalized to the value of initial (F0) fluorescence yield (c and d). The measurements were taken in the presence of 5·10–5 M DCMU added 1 min before switching on the actinic light; the actinic light was 620 nm (40 µmol photons·m–2·s–1). ↑ and ↓, switching the actinic light on and off; chlorophyll content in samples – 10 µg/ml.
Wild type Synechocystis PCC 6803 cells were previously shown to exhibit only a slight decrease in fluorescence yield after dark adaptation, i.e. their photosynthetic apparatus undergoes practically no changes typical for the transition to State 2 [26]. Variable fluorescence yield, measured in the current study for dark-adapted wild type cells, changes insignificantly after 20 min illumination (Fig. 3), which is in good agreement with a minimal PSII fluorescence increase in 77 K fluorescence emission spectra observed for wild type cells after illumination [36]. These data indicate that the state of the photosynthetic apparatus of these cells is close to State 1. This is also indicated by lack of changes in the SDBMIB/SDCMU value for dark- or light-adapted wild type cells (Fig. 2). A high variable fluorescence yield of dark-adapted SDH– mutant cells and its insignificant change after 20 min of light adaptation (Fig. 3) indicate that the photosynthetic apparatus of these cells are in State 1 in the dark and under light conditions. State 1 of the SDH– mutant photosynthetic apparatus is confirmed by a lack of changes in 77 K fluorescence spectra [36], even though, from the SDBMIB/SDCMU ratio the PQ pool of the SDH– mutant is more reduced after 20 min illumination than after dark adaptation (Fig. 2).
A low yield of variable fluorescence in the presence of DCMU, which is characteristic of dark-adapted Ox– cells, corresponds to a low SDBMIB/SDCMU ratio, indicating a reduced PQ pool (Fig. 2). Together these facts indicate State 2 of the photosynthetic apparatus for dark-adapted Ox– cells. An increase in the induction of fluorescence measured in the presence of DCMU (Fig. 3) and a significant increase in the relative yield of PSII 77 K fluorescence after illumination of Ox– cells [36] indicate a change in the photosynthetic apparatus organization for dark-adapted Ox– cells during a transition from State 2 to State 1 in the light. However, a significant increase in the fluorescence yield (Fig. 3, b and d) in light-adapted Ox– cells is not related to a notable change in PQ pool redox state (Fig. 2). These data suggest that the PQ pool redox state may not be a direct signal for regulation of PSII activity during photosynthetic apparatus reorganization during transitions between States 1 and 2. Probably, there are reactions indirectly associated with the PQ pool redox state that could change PSII activity.
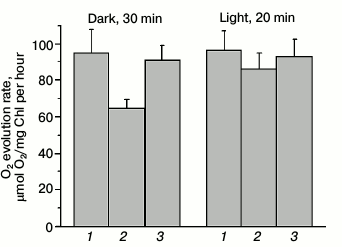
Photochemical activity of PSII measured by oxygen evolution. A low yield of PSII fluorescence in dark-adapted Ox– cells corresponded to a lower O2 evolution rate as compared to wild type and SDH– mutant cells (Fig. 4). The oxygen evolution rate in Ox– mutant cells was 30% lower than that of wild type cells (Fig. 4), while the oxygen evolution rate for SDH– mutant cells was only 3-5% lower or practically the same as for wild type cells.
Fig. 4. PSII oxygen evolution rate for wild type Synechocystis cells (1) and Ox– (2) and SDH– mutants (3) incubated for 30 min in the dark or illuminated for 20 min by white light, 25 µmol photons·m–2·s–1, with the natural electron donor (H2O) and acceptor (CO2). The actinic light (800 µmol photons·m–2·s–1) was switched on 3 min after placing the cells in the measuring cuvette; a measurement was taken during the next 3-4 min, and then the light was turned off. The diagram is based on mean parameter values ± SD from three experiments.
A low rate of O2 evolution and a low yield of variable fluorescence for Ox– cells indicated that at least 30% of PSII reaction centers (compared to wild type) are inactive, and the donor side of PSII may also be inactivated within them. Adaptation to low-intensity white light illumination (25 µmol photons·m–2·s–1) activated PSII in Ox– cells, indicated by an increase in oxygen evolution rate by approximately 30% as compared to O2 evolution rate of dark-adapted Ox– cells (Fig. 4). Apparently, intense actinic light kept a portion of PSII reaction centers of Ox– mutant in a photochemically inactive state, while a low illumination activated the PSII reaction centers. To evaluate the functional state of PSII reaction centers in Synechocystis cells in more detail, we analyzed variable fluorescence kinetics under high-intensity pulsed light in the sub-millisecond range in the absence of DCMU with a device measuring fluorescence induction and relaxation (FIRe).
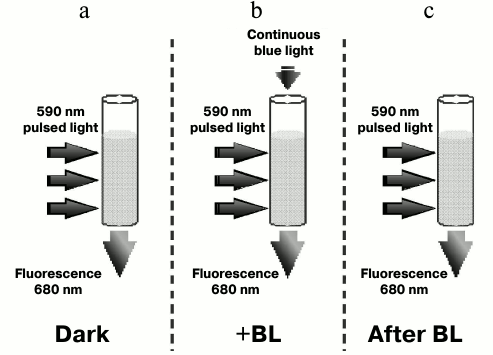
Fluorescence induction and relaxation kinetics for Synechocystis cells measured with the FIRe-system pulse fluorometer. Figure 5 shows the experimental setup for measuring fluorescence induction and relaxation kinetics in wild type and Ox– mutant cells with excitation pulsed light of 590 nm. For measurements, short pulses of light were used, absorbed by PBS (590 nm, 1 W/cm2, 0.1 ms) or chlorophyll and carotenoids (450 nm, 1 W/cm2, 0.3 ms) and low (20 µmol photons·m–2·s–1) additional continuous light of 470 nm (hereinafter referred to as BL). These illumination parameters minimized the possibility of photoinhibition and non-photochemical fluorescence quenching due to interaction of phycobilisomes with the orange carotenoid protein, and they allowed measurement of fluorescence changes related mainly to the state transitions of the cyanobacterial photosynthetic apparatus.
Fig. 5. Schematic representation of the experiment measuring fluorescence induction and relaxation kinetics in Synechocystis cells excited by 590 nm pulsed light in the dark (a), excited by pulsed light with the background low (20 µmol·m–2·s–1) continuous 470 nm illumination (+BL) (b), and excited by pulsed light immediately after switching off continuous 470 nm illumination (c).
Photophysiological parameters of dark-adapted wild type and mutant Synechocystis cells measured with 590 nm excitation (Table 1). Table 1 shows fluorescence parameters of dark-adapted Synechocystis cells measured under 590 nm excitation. Since the PQ pool of the Ox– mutant, lacking terminal oxidases, is reduced (Fig. 2), the re-oxidation rate for the quinone acceptor QA was extremely low. This is evident from the value of the re-oxidation time constant (τQA), which, for the Ox– mutant, exceeded the corresponding parameters of wild type and SDH– mutant cells by 4-5 times (Table 1). Under BL illumination, all three cell types were characterized by a similar τQA values (Table 1). The low QA– reoxidation rate with the reduced PQ pool for the Ox– mutant suggests a higher portion of PSII reaction centers in which the primary quinone acceptor is reduced (QA–), i.e. closed PSII reaction centers, due to reduced electron transfer from QA– to QB [37]. An increase in QA– oxidation rate under BL illumination of Ox– cells may indicate an acceleration of linear electron transfer.
Table 1. Parameters determined from
fluorescence induction and relaxation kinetics for wild type
Synechocystis cells (WT), the mutant lacking terminal oxidases
(Ox–), and the mutant lacking succinate dehydrogenase
(SDH–), adapted in the dark for 30 min.
Measurements were taken in saturating single turnover flash (STF) mode;
excitation light was 590 nm (0.1 ms, 1 W/cm2), in
the presence (+BL) or in the absence (–BL) of continuous actinic
light (BL, 470 nm, 20 µmol
photons·m–2·s–1).
τQA (ms) is the time constant of the
Q–A reoxidation reaction;
Fv/Fm, quantum yield of the primary PSII
photochemical reaction; ρ, a parameter reflecting energy exchange
between PSII reaction centers; σ590, PSII functional
absorption cross-section for 590 nm light. Mean values ± SD
were calculated from the results of 6-7 independent experiments. The
optical density of the cell suspension was no higher than 0.02 at the
maximum of chlorophyll absorption
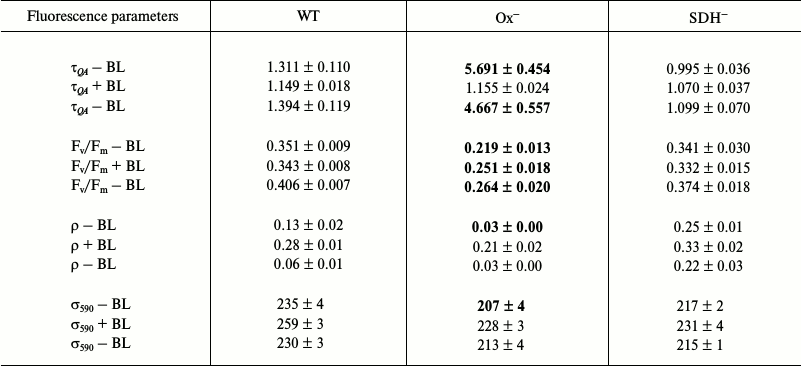
Long-term (30 min) incubation of Synechocystis cells in the dark caused deactivation of FNR and Calvin cycle enzymes [38-41], so short pulses of intense light could induce cyclic and reverse reactions in PSI [38]. Some authors suppose that short pulses of intense light may induce reverse electron transport in PSII as well, from quinone acceptors to P680+ during dark intervals between flashes [42, 43]. Then, BL illumination (approximately 25 s against the background of a series of 50 intense light pulses (Fig. 5b)) could activate linear electron transport in the photosystems. It should be noted that activation of FNR by light occurs within a few seconds [39]. However, in contrast to the Ox– mutant, BL illumination of the wild type and SDH– mutant cells had practically no effect on the τQA value (Table 1), and its low values indicated a high rate of QA– reoxidation even in dark-adapted cells with oxidized PQ pool. This suggests a large portion of open (QA) PSII reaction centers is present in wild type and SDH– cells.
For the Ox– mutant, after switching off continuous blue background light, the QA– reoxidation time constant increased by 4 times, while for wild type and SDH– mutant the value of this parameter increased only slightly compared to values measured after dark adaptation. These data confirm that short single light pulses used in measurements do not support linear electron transfer.
The ρ parameter reflects the probability for transfer of absorbed energy between PSII reaction centers; there is a positive correlation between high values of ρ and the efficiency of photochemical processes [37]. A change in fluorescence of dark-adapted cells showed low values of ρ parameter for Ox– cells: 4 times lower than that of wild type and 8 times lower than that of SDH– mutant (Table 1). Low ρ values and a low rate of QA– reoxidation (high τQA) for Ox– corresponded to a low quantum yield of the primary photochemical reaction (Fv/Fm), which was 40% lower compared to that of wild type and SDH– (Table 1). Moreover, based on low oxygen evolution rate, at least 30% of PSII reaction centers in Ox– cells are in an inactivated state. In presence of BL, a 7-fold increase of ρ was observed for Ox– cells (Table 1) and also an increase in QA– reoxidation rate (by 5 times) and a slight increase of the Fv/Fm parameter (~15%). A lower QA– reoxidation rate and lower ρ and Fv/Fm parameter values for Ox– compared to those for wild type and SDH– indicate a larger portion of closed and photochemically inactive (not exhibiting variable fluorescence) PSII reaction centers in the Ox– mutant. Slow QA– reoxidation in dark-adapted Ox– cells (when the PQ pool is reduced) and acceleration of this reactions under continuous blue light (BL), which activates linear electron transport, suggest a higher probability of back (or cyclic) reactions in PSII under pulsed fluorescence excitation. In wild type and SDH– cells (with reduced PQ pool) fast QA– reoxidation after dark adaptation and a lack of changes in the rate of this reaction under BL suggest a low probability of back (or cyclic) reactions in PSII under intense pulse illumination of these cells. Some authors suppose that back (or cyclic) reactions may cause nonradiative dissipation of absorbed energy in PSII reaction centers [43-46]. In this regard, a low quantum yield of the primary photochemical reaction (Fv/Fm) in Ox– may be caused by a large probability of back (or cyclic) reactions in PSII in the absence of continuous illumination.
A slight increase in quantum yield of the primary photochemical reaction (Fv/Fm) under BL illumination in the Ox– mutant corresponded to a 10% increase in PSII functional absorption cross-section for 590 nm light (and a 9% decrease in F0), accompanied by some kind of PSII activation (~15%) by 590 nm light under continuous BL illumination. BL illumination of wild type and SDH– cells also cause an increase in σ590 by 10 and 5%, respectively (and a 5% increase in F0). However, this increase was not accompanied by an increase in the Fv/Fm parameter and QA– re-oxidation rate (Table 1). Thus, an increase in σ590 and QA– re-oxidation rate under BL caused a moderate PSII activation in the Ox– mutant, whose photosynthetic apparatus was in State 2. An increase in σ590 in the absence of QA– reoxidation acceleration did not cause PSII activation in wild type and SDH– mutant, whose photosynthetic apparatus was in State 1.
Photophysiological parameters of dark-adapted wild type and mutant Synechocystis cells measured with 450 nm exciting light (Table 2). Table 2 shows photophysiological parameters of dark-adapted Synechocystis cells measured under 450 nm excitation (1 W/cm2), which is absorbed by chlorophyll and carotenoids.
Table 2. Parameters determined from
fluorescence induction and relaxation kinetics for wild type
Synechocystis cells (WT) and Ox– and
SDH– mutants adapted to the dark for 30 min.
Measurements were taken in saturating single turnover flash (STF) mode;
excitation light was 450 nm (0.3 ms, 1 W/cm2) in
the presence (+BL) or in the absence (–BL) of continuous actinic
light (BL, 470 nm, 20 µmol
photons·m–2·s–1).
Parameter σ450 is the PSII functional absorption
cross-section for 450 nm light. The remaining parameter
designations are the same as in Table 1. Mean values ± SD
were calculated from the results of 6-7 independent experiments. The
optical density of the cell suspension was no higher than 0.02 at the
maximum of chlorophyll absorption
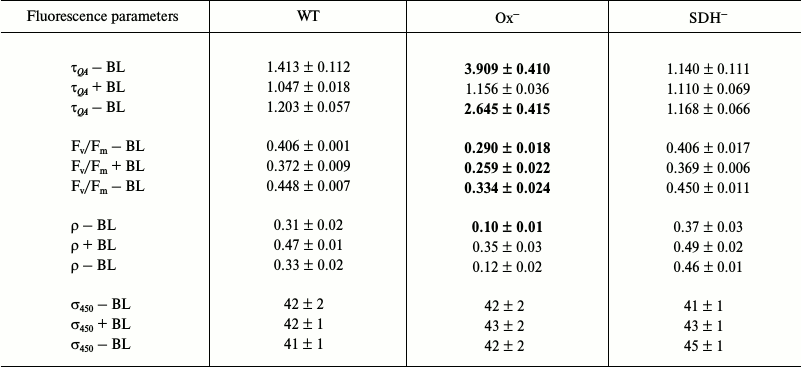
Differences of Ox– mutant from wild type and SDH– cells are less pronounced under 450 nm excitation than differences under 590 excitation (Tables 1 and 2). This may be related to different values of PSII functional absorption cross-section, which is 5 times lower for blue light of 450 nm (σ450) than that for 590 nm light (σ590) absorbed by phycobilisomes (Tables 1 and 2). When using 450 nm pulses, all types of Synechocystis cells showed higher values of ρ and Fv/Fm parameters compared to those measured under 590 nm excitation. This may be explained by the fact that during QA reduction by 450 nm light pulses, the portion of photochemically active (i.e. with variable fluorescence) PSII reaction centers was higher than under excitation by 590 nm pulses, which is also related to a low σ450 value. Despite this, the Ox– mutant was characterized by a 3.0-3.5 times lower ρ and a 2.5-3.0 times higher τQA compared to those of wild type and SDH– mutant (Table 2). Under these conditions, τQA values showed practically no changes for wild type and SDH– compared to those measured under 590 nm light.
Under BL background QA– reoxidation rate and ρ value increased 3.5 times for Ox– cells, and approximately 1.5 times for wild type cells, and less than 1.5 times for SDH–. However, unlike the case of 590 nm excitation, measurements with 450 nm exciting light with the BL background did not show even a slight PSII activation in the Ox– mutant (Tables 1 and 2). In all the cell types including Ox–, a 8-10% decrease in the Fv/Fm parameter was observed under 450 nm excitation against the BL background (Table 2). BL illumination had virtually no effect on 450 nm light absorption efficiency for all the Synechocystis strains, as shown by the absence of measureable changes in σ450 parameter (Table 2). However, as noted previously, under fluorescence excitation with 590 nm light, BL illumination of the Ox– mutant caused an increase in the Fv/Fm parameter and in PSII functional absorption cross-section σ590 (Table 1). In this regard, we suggest that BL (470 nm), mainly absorbed by chlorophyll and carotenoids (light 1), could cause changes in the photosynthetic apparatus of the Ox– mutant, leading to an increase in absorption efficiency for “phycobilisome” (absorbed by PBS) 590 nm light by reaction centers of PSII and its activation. An increase in efficiency of energy transfer from PBS to PSII is assumed to be related to photosynthetic apparatus reorganization during the transition from State 2 to State 1 [1, 17, 18]. Wild type and SDH– cells, with the photosynthetic apparatus in State 1 (or a similar one) after dark adaptation, did not express PSII activation in response to BL illumination under excitation by 450 or 590 nm light. After turning BL off, an increase in Fv/Fm was observed for all cell types (Tables 1 and 2), accompanied by a noticeable slowing of QA– reoxidation and decrease in ρ, which may be related to termination of linear electron transport in the PSII acceptor site. In addition, turning off BL could cause some reactivation of PSII reaction centers due to a decrease in σ590 for all cell types (Table 1).
Photophysiological parameters of light-adapted wild type and mutant Synechocystis cells measured with 590 and 450 nm exciting light (Tables 3 and 4, respectively). Low illumination (25 µmol photons·m–2·s–1, 20 min) of dark-adapted Synechocystis cells activates FNR and Calvin cycle enzymes [38-41], which can activate linear electron transport. Although, in general, changes in photophysiological parameters of wild type and mutant cells incubated under low (25 µmol photons·m–2·s–1) white light are similar to those observed for dark-adapted cells.
Table 3. Parameters determined from
fluorescence induction and relaxation kinetics for wild type
Synechocystis cells (WT) and Ox– and
SDH– mutants adapted to white light
(25 µmol
photons·m–2·s–1) for
20 min. Measurements were taken in saturating single turnover
flash (STF) mode; excitation light was 590 nm (0.1 ms,
1 W/cm2). The conditions for experiments and
designations are the same as in Table 1
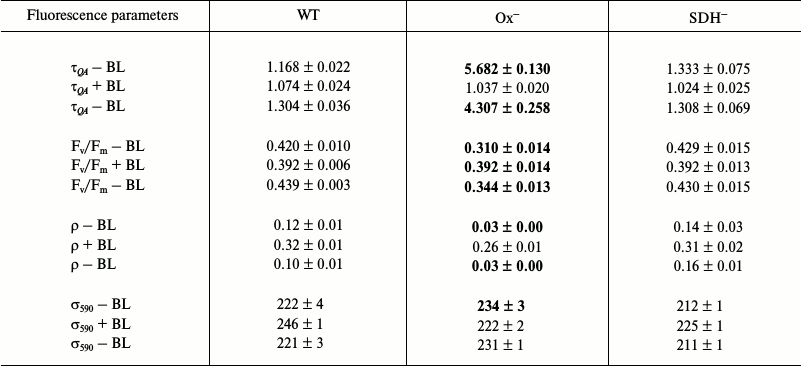
Table 4. Parameters determined from
fluorescence induction and relaxation kinetics for wild type
Synechocystis cells (WT) and Ox– and
SDH– mutants adapted to white light
(25 µmol
photons·m–2·s–1) for
20 min. Measurements were taken in saturating single turnover
flash (STF) mode; excitation light was 450 nm (0.3 ms,
1 W/cm2). The conditions for experiments and
designations are the same as in Table 2
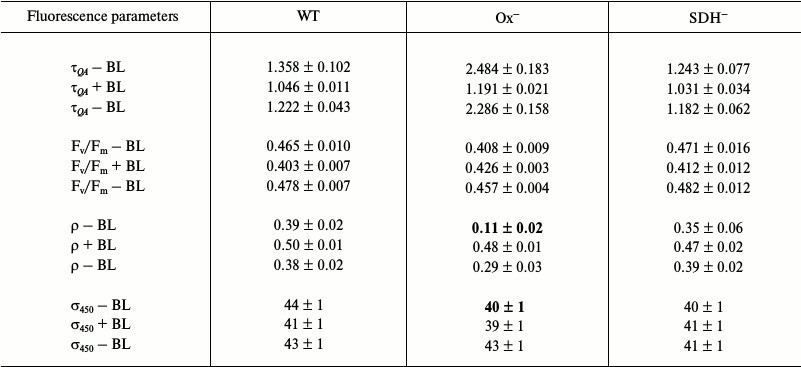
The QA– reoxidation time constants and ρ parameters measured with 590 nm exciting light for light-adapted Ox– and wild type cells are not very differed from those for dark-adapted cells (Tables 1 and 3). This is due to a very slight change in PQ pool redox state for Ox– and wild type cells (Fig. 2) and a high value of functional absorption section σ590. Apparently, slow QA– reoxidation and low ρ value for Ox– cells were determined by reduced PQ pool in addition to the absence of linear electron transport, which stopped when cells were transferred from continuous white light to a cuvette for measurements with intense 590 nm light pulses (Fig. 5a and Table 3; top τQA – BL and ρ – BL). Slow QA– reoxidation and a low ρ value for Ox– cells suggest a high probability of back (or cyclic) reaction in PSII of Ox– mutant under illumination with intense 590 nm light pulses. In wild type and SDH– cells with oxidized PQ, QA– re-oxidation rate and ρ value were 4-5 times higher than that for Ox– (top τQA – BL and ρ – BL; Table 3), suggesting a low probability for back (or cyclic) reactions in PSII even under excitation with 590 nm pulses. The quantum yield of the primary photochemical reaction Fv/Fm in light-adapted Ox– cells was 25% lower compared to Fv/Fm of wild type cells (top Fv/Fm – BL; Table 3), that may also be related to a higher probability for back (or cyclic) reactions in PSII of Ox– cells. For the SDH– mutant, QA– re-oxidation rate and ρ value slightly decreased after light adaptation (Tables 1 and 3), that may be explained by PQ pool reduction due to PSII photochemical activity in the light; in the dark, the PQ pool in the SDH– mutant is oxidized due to the absence of succinate dehydrogenase (Fig. 1).
BL illumination of wild type and SDH– Synechocystis cells caused an insignificant increase in QA– oxidation rate and an increase in ρ of approximately 2.0-2.5 fold, while for the Ox– mutant these values increased 5- and 8-fold, respectively (Table 3). However, unlike the dark-adapted cells, BL illuminated cells did not cause σ590 increase in Ox– mutant pre-incubated in the light (σ590 + BL; Table 3). Despite this, an increase in Fv/Fm was observed for Ox– cells under BL with 590 nm pulses (Table 3). It should be noted that a 10% increase in σ590 for Ox– cells already occurred during adaptation (20 min) of this mutant to low white light (top σ590 – BL; Tables 1 and 3). However, a high value of functional absorption cross-section σ590 and reduced pool in Ox– cells set a low QA– reoxidation rate and a low ρ value even after light adaptation, indicating a large portion of closed PSII centers under 590 nm excitation. Continuous BL caused an acceleration of QA– reoxidation and a 25% increase in Fv/Fm for Ox– cells, indicating activation of a portion of PSII reaction centers (Table 3). Thus, the ratio of absorbed energy to QA– re-oxidation rate, which is influenced by PQ redox state, is apparently determined by the functional state of PSII reaction centers.
The least significant variation in fluorescent parameters for Ox– mutant cells from wild type parameters was observed after light adaptation with 450 nm excitation, characterized by a low σ450 value as compared to σ590. Ox– cells incubated in the light showed only a 2 times lower QA– reoxidation rate and only a 12% decrease in Fv/Fm compared to wild type cells (top τQA – BL and Fv/Fm – BL; Table 4), indicating an increase in the portion of open and photochemically active PSII reaction centers of Ox– mutant after light adaptation. When measuring fluorescence with 450 nm light pulses and under BL illumination, only a slight Fv/Fm increase (~4 %) was observed, and σ450 remained unchanged (Tables 2 and 4).
Light adaptation (20 min) of Synechocystis cells first incubated in the dark caused an increase in quantum yield of the primary photochemical reaction (Fv/Fm) in PSII of all studied strains, as found during fluorescence excitation by both 590 nm (top Fv/Fm – BL; Tables 1 and 3) and 450 nm (top Fv/Fm – BL; Tables 2 and 4) light pulses. The maximum increase in PSII activity after light adaptation was observed for Ox– lacking the terminal oxidases: Fv/Fm value increased by 40% under both 590 nm (top Fv/Fm – BL; Tables 1 and 3) and 450 nm (top Fv/Fm – BL; Tables 2 and 4) excitation light. For wild type and SDH–, the increase in Fv/Fm was less pronounces (15-20% after light adaptation). An increase in Fv/Fm in Ox– cells adapted to white light corresponded to a 30% increase in PSII O2 evolution rate (Fig. 4), indicating PSII activation (supposedly the donor site) under illumination with low continuous white light. Moreover, for Ox– mutant after prolonged (20 min) light adaptation, a 10% increase in functional absorption cross-section was found (top σ590 – BL; Tables 1 and 3); no such change is observed for wild type and SDH–. The results of measurements of fluorescence kinetics in the presence of DCMU (Fig. 3) and of 77 K fluorescence spectra [36] also revealed maximum changes in fluorescence parameters for Ox– mutant after prolonged light adaptation. It should be noted that for PSII reactivation in dark-adapted Ox– (to wild type level) in the presence of DCMU, a few tens of seconds of continuous low illumination are enough [36]. Changes in fluorescence parameters similar to those observed during the study of Ox– cells after light adaptation are usually associated with changes occurring in the cyanobacterial photosynthetic apparatus during a transition from State 2 to State 1 under the action of light [7, 8, 10, 11, 16-18].
Thus, incubating cyanobacterial cells in the dark caused inactivation of PSII, which was related to the PQ pool redox state. Reversible inactivation of PSII reaction centers was earlier shown to occur during the initial state of photoinhibition not related to D1 protein degradation; one of the reasons for PSII reversible inactivation is accumulation of reducing equivalents in PSII acceptor site [44, 45, 47]. At the same time, cyanobacteria, unlike higher plants, are known to accumulate reducing equivalents in the PSII acceptor site even in the dark, since their thylakoid membranes possess functioning respiratory dehydrogenases that reduce the PQ pool [10-12, 23]. Due to this, as well as the absence of respiratory oxidases in the Ox– mutant (Fig. 1), the PQ pool of this mutant is completely reduced after incubation in the dark [24]. Some authors suppose that during inhibition of electron transfer from QA– to QB, when the PQ pool is reduced, alternative pathways may appear for electron transfer from quinone acceptors (QA– and QB–) to P680+ in PSII, accompanied by a decrease in fluorescence thus protecting PSII from photoinhibition [43-46]. It is also supposed that inhibition of the PSII donor site caused by thylakoid lumen acidification may alter the redox potential of quinone acceptors, which also causes charge recombination in reaction center between QA– and P680+ [48]. These processes could cause reversible fluorescence quenching for PSII reaction centers in dark-adapted cyanobacteria (State 2 of photosynthetic apparatus).
In addition, our study has shown continuous illumination with low-intensity blue light BL (~25 s) or low-intensity white light used for prolonged (20 min) adaptation caused an increase in absorption efficiency for 590 nm light by PSII reaction centers in Ox– mutant cells, accompanied by the activation of this photosystem; no changes in absorption efficiency for 450 nm light were detected. Such changes in cyanobacterial fluorescence reflect a reorganization of the photosynthetic apparatus during a transition from State 2 to State 1, connected to a shift of PBS in relation to PSII core complex [1, 17, 18].
Thus, the method of microsecond pulse fluorometry revealed two processes that could participate in PSII activity changes in response to dark or light in cyanobacteria. One is reversible PSII inactivation during dark adaptation, affected by the redox state of the PSII acceptor site. Another is found under low illumination as an increase in absorption efficiency for “phycobilisome” (absorbed by PBS) light by PSII reaction centers. It is highly likely that a change in PSII reaction center functional state is accompanied by conformational rearrangements that cause PBS movements relative to PSII core complex.
Acknowledgements
This research was supported by the Russian Foundation for Basic Research (project No. 14-04-00148) and by the Russian Science Foundation (project No. 14-17-00451).
REFERENCES
1.Mullineaux, C. W., and Holzwarth, A. R. (1990) A
proportion of photosystem II core complexes are decoupled from the
phycobilisome in light-state 2 in the cyanobacterium
Synechococcus 6301, FEBS Lett., 260, 245-248.
2.Bonaventura, C., and Myers, J. (1969) Fluorescence
and oxygen evolution from Chlorella pyrenoidosa, Biochim.
Biophys. Acta, 189, 366-383.
3.Murata, N. (1969) Control of excitation transfer in
photosynthesis: I. Light-induced change of chlorophyll a fluorescence
in Porphyridium cruentum, Biochim. Biophys. Acta,
172, 242-251.
4.Fork, D. C., and Satoh, K. (1983) State
I–state II transitions in the thermophilic blue-green alga
(cyanobacterium) Synechococcus lividus, Photochem.
Photobiol., 37, 421-427.
5.Allen, J. F. (2003) State transitions –
a question of balance, Science, 299, 1530-1532.
6.Mullineaux, C. W., and Allen, J. F. (1990) State
1–state 2 transitions in the cyanobacterium Synechococcus
6301 are controlled by the redox state of electron carriers between
photosystems I and II, Photosynth. Res., 23, 297-311.
7.Mao, H.-B., Li, G.-F., Ruan, X., Wu, Q.-Yu, Gong,
Y.-D., Zhang, X.-F., and Zhao, N.-M. (2002) The redox state of
plastoquinone pool regulates state transitions via cytochrome
b6/f complex in Synechocystis sp. PCC
6803, FEBS Lett., 519, 82-86.
8.Mullineaux, C. W., and Allen, J. F. (1988)
Fluorescence induction transients indicate dissociation of photosystem
II from the phycobilisome during the state 2 transition in the
cyanobacterium Synechococcus 6301, Biochim. Biophys.
Acta, 934, 96-107.
9.Rakhimberdieva, M. G., Boichenko, V. A.,
Karapetyan, N. V., and Stadnichuk, I. N. (2001) Interaction of
phycobilisomes with photosystem II dimers and photosystem I monomers
and trimers in the cyanobacterium Spirulina platensis,
Biochemistry, 40, 15780-15788.
10.Mullineaux, C. W., and Allen, J. F. (1986) The
state 2 transition in the cyanobacterium Synechococcus 6301 can
be driven by respiratory electron flow into the plastoquinone pool,
FEBS Lett., 205, 155-160.
11.Huang, Ch., Yuan, X., Zhao, J., and Bryant, D. A.
(2003) Kinetic analyses of state transitions of the cyanobacterium
Synechococcus sp. PCC 7002 and its mutant strains impaired in
electron transport, Biochim. Biophys. Acta, 1607,
121-130.
12.Mi, H., Endo, T., Schreiber, U., Ogawa, T., and
Asada, K. (1992) Electron donation from cyclic and respiratory flows to
the photosynthetic intersystem chain is mediated by pyridine nucleotide
dehydrogenase in the cyanobacterium Synechocystis sp. PCC 6803,
Plant Cell Physiol., 33, 1233-1237.
13.Howitt, C. A., Smith, G. D., and Day, D. A.
(1993) Cyanide-insensitive oxygen uptake and pyridine nucleotide
dehydrogenases in the cyanobacterium Anabaena PCC 7120,
Biochim. Biophys. Acta, 1141, 313-320.
14.Cooley, J. W., Howitt, C. A., and Vermaas, W. F.
J. (2000) Succinate:quinol oxidoreductase in the cyanobacterium
Synechocystis sp. strain PCC 6803: presence and function in
metabolism and electron transport, J. Bacteriol., 182,
714-722.
15.Pils, D., and Schmetterer, G. (2001)
Characterization of three bioenergetically active respiratory terminal
oxidases in the cyanobacterium Synechocystis sp. strain PCC
6803, FEMS Lett., 203, 217-222.
16.Meunier, P. C., Colon-Lopez, M. S., and
Sherman, L. A. (1997) Temporal changes in state transitions and
photosystem organization in the unicellular, diazotrophic
cyanobacterium Cyanothece sp. ATCC 51 142, Plant
Physiol., 115, 991-1000.
17.Kirilovsky, D. (2014) Modulating energy arriving
at photochemical reaction centers: orange carotenoid protein-related
photoprotection and state transitions, Photosynth. Res.,
126, 3-17.
18.Mullineaux, C. W., Tobin, M. J., and Jones, G. R.
(1997) Mobility of photosynthetic complexes in thylakoid membranes,
Nature, 390, 421-424.
19.Schluchter, W. M., Shen, G., Zhao, J., and
Bryant, D. A. (1996) Characterization of psaI and psaL mutants of
Synechococcus sp. strain PCC 7002: a new model for state
transitions in cyanobacteria, Photochem. Photobiol., 64,
53-66.
20.Ivanov, A. G., Krol, M., Sveshnikov, D., Selstam,
E., Sandstrom, St., Koochek, M., Park, Y.-I., Vasil’ev, S.,
Bruce, D., Oquist, G., and Huner, N. P. A. (2006) Iron deficiency in
cyanobacteria causes monomerization of photosystem I trimers and
reduces the capacity for state transitions and the effective absorption
cross section of photosystem I in vivo, Plant Physiol.,
141, 1436-1445.
21.Zhang, R., Xie, J., and Zhao, J. (2009) The
mobility of PSI and PQ molecules in Spirulina platensis cells
during state transition, Photosynth. Res., 99,
107-113.
22.McConnell, M. D., Koop, R., Vasil’ev, S.,
and Bruce, D. (2002) Regulation of the distribution of chlorophyll and
phycobilin-absorbed excitation energy in cyanobacteria. A
structure-based model for the light state transition, Plant
Physiol., 130, 1201-1212.
23.Cooley, J. W., and Vermaas, W. F. J. (2001)
Succinate dehydrogenase and other respiratory pathways in thylakoid
membranes of Synechocystis sp. strain PCC 6803: capacity
comparisons and physiological function, J. Bacteriol.,
183, 4251-4258.
24.Howitt, C. A., Cooley, J. W., Wiskich, J. T., and
Vermaas, W. F. J. (2001) A strain of Synechocystis sp.
PCC 6803 without photosynthetic oxygen evolution and respiratory oxygen
consumption: implications for the study of cyclic photosynthetic
electron transport, Planta, 214, 46-56.
25.Ma, W., Mi, H., and Shen, Yu. (2010) Influence of
the redox state of QA on phycobilisome mobility in the
cyanobacterium Synechocystis sp. strain PCC6803, J.
Luminesc., 130, 1169-1173.
26.Campbell, D., Hurry, V., Clarke, A. K.,
Gustafsson, P., and Oquist, G. (1998) Chlorophyll fluorescence analysis
of cyanobacterial photosynthesis and acclimation, Microbiol. Mol.
Biol. Rev., 62, 667-683.
27.Howitt, C. A., and Vermaas, W. F. J. (1998)
Quinol and cytochrome oxidases in the cyanobacterium
Synechocystis PCC 6803, Biochemistry, 37,
17944-17951.
28.Rippka, R., Deruelles, J., Waterbury, J. B.,
Herdman, M., and Stanier, R. Y. (1979) Generic assignments, strain
histories and properties of pure cultures of cyanobacteria, J. Gen.
Microbiol., 111, 1-61.
29.Lichtenthaler, H. K. (1987) Chlorophylls and
carotenoids: pigments of photosynthetic biomembranes, Methods
Enzymol., 148C, 350-382.
30.Schreiber, U., Schliwa, U., and Bilger, W. (1986)
Continuous recording of photochemical and non-photochemical chlorophyll
fluorescence quenching with a new type of modulation fluorometer,
Photosynth. Res., 10, 51-62.
31.Berry, S., Schneider, D., Vermaas, W. F. J., and
Roegner, V. (2002) Electron transport routes in whole cells of
Synechocystis sp. strain PCC 6803: the role of the cytochrome
bd-type oxidase, Biochemistry, 41, 3422-3429.
32.Gorbunov, M. Y., and Falkowski, P. G. (2005) in
Photosynthesis. Fundamental Aspects to Global Perspectives: 13th
Int. Congr. on Photosynthesis (Van der Est, A., and Bruce, D.,
eds.) Alliance Communication Group, Lawrence, Kansas, pp.
1029-1031.
33.Kolber, Z. S., Prasil, O., and Falkowski, P. G.
(1998) Measurements of variable chlorophyll fluorescence using fast
repetition rate techniques: defining methodology and experimental
protocols, Biochim. Biophys. Acta, 1367, 88-106.
34.Joliot, A., and Joliot, P. (1964) Kinetic study
of the potochemical reaction liberating oxygen during photosynthesis,
CR Hebd. Seances Acad. Sci., 258, 4622-4625.
35.Lavergne, J., and Trissl, Y. W. (1995) Theory of
fluorescence induction in photosystem II: derivation of analytical
expressions in a model including exciton-radical-pair equilibrium and
restricted energy transfer between photosynthetic units, Biophys.
J., 68, 2474-2492.
36.Bolychevtseva, Y. V., Kuzminov, F. I., Elanskaya,
I. V., Gorbunov, M. Y., and Karapetyan, N. V. (2015) Photosystem
activity and state transitions of the photosynthetic apparatus in
cyanobacterium Synechocystis PCC 6803 mutants with different
redox state of the plastoquinone pool, Biochemistry (Moscow),
80, 50-60.
37.Zhu, X.-G., Govindjee, Baker, N. R.,
D’Sturler, E., Ort, D. R., and Long, S. P. (2005) Chlorophyll a
fluorescence induction kinetics in leaves predicted from a model
describing each discrete step of excitation energy and electron
transfer associated with photosystem II, Planta, 223,
114-133.
38.Talts, E., Oja, V., Ramma, H., Rasulov, B.,
Anijalg, A., and Laisk, A. (2007) Dark inactivation of ferredoxin-NADP
reductase and cyclic electron flow under far-red light in sunflower
leaves, Photosynth. Res., 94, 109-120.
39.Pschorn, R., Ruhle, W., and Wild, A. (1988)
Structure and function of ferredoxin-NADP+-oxidoreductase,
Photosynth. Res., 17, 217-229.
40.Pelroy, R. A., and Bassham, J. A. (1972)
Photosynthetic and dark carbon metabolism in unicellular blue-green
algae, Arch. Microbiol., 86, 25-38.
41.Pelroy, R. A., Levine, G. A., and Bassham, J. A.
(1976) Kinetics of light-dark CO2 fixation and glucose
assimilation by Aphanocapsa 6714, J. Bacteriol.,
128, 633-643.
42.Keren, N., Berg, A., Van Kan, P. J. M., Levanon,
H., and Ohad, I. (1997) Mechanism of photosystem II photoinactivation
and D1 protein degradation at low light: the role of back electron
flow, Proc. Natl. Acad. Sci. USA, 94, 1579-1584.
43.Ohad, I., Berg, A., Berkowicz, S. M., Kaplan, A.,
and Keren, N. (2011) Photoinactivation of photosystem II: is there more
than one way to skin a cat? Physiol. Plant., 142,
79-86.
44.Van Wijk, K. J., and Van Hasselt, Ph. R. (1993)
Photoinhibition of photosystem II in vivo is preceded by
down-regulation through light-induced acidification of the lumen:
consequences for the mechanism of photoinhibition in vivo,
Planta, 189, 359-368.
45.Leitsch, J., Schnettger, B., Critchley, Ch., and
Krause, G. H. (1994) Two mechanisms of recovery from photoinhibition
in vivo: reactivation of photosystem II related and unrelated to
D1-protein turnover, Planta, 194, 15-21.
46.Ivanov, A. G., Sane, P. V., Hurry, V., Oquist,
G., and Huner, N. P. A. (2008) Photosystem II reaction centre
quenching: mechanisms and physiological role, Photosynth. Res.,
98, 565-574.
47.Andersson, B., and Barber, J. (1996) in
Photosynthesis and the Environment. Advances in Photosynthesis and
Respiration (Baker, N. R., ed.) Kluwer Academic Publishers,
Springer, Dordrecht, Vol. 5, pp. 101-121.
48.Horton, P., Ruban, A. V., and Walters, R. G.
(1994) Regulation of light harvesting in green plants, Plant
Physiol., 106, 415-420.