Immunogenicity of Human Interferon-Beta-Containing Pharmaceuticals
V. D. Nazarov1*, S. V. Lapin1, A. V. Mazing1, E. P. Evdoshenko2, and A. A. Totolian1,3
1Center for Molecular Medicine, Pavlov First Saint Petersburg State Medical University, 197022 St. Petersburg, Russia; E-mail: Nazarov19932@mail.ru2Saint Petersburg Center of Multiple Sclerosis and Autoimmune Diseases Clinical Hospital No. 31, 197110 St. Petersburg, Russia; E-mail: centerms@gmail.com
3Saint Petersburg Pasteur Research Institute of Epidemiology and Microbiology (St. Petersburg Pasteur Institute), 197101 St. Petersburg, Russia; E-mail: pasteur@pasteurorg.ru
* To whom correspondence should be addressed.
Received June 13, 2016; Revision received August 29, 2016
Multiple sclerosis is a severe autoimmune disease with inflammatory component that continues to be resistant to treatment. One of the approaches retarding its progression is based on using nonspecific therapy with human interferon-beta (IFN-β)-containing pharmaceuticals. Neutralizing antibodies (NAbs) against genetically engineered pharmaceuticals developed by the patient’s immune system, which reduce their therapeutic and biological activity, pose a serious problem. Cell lines sensitive to IFN-β activity also quantifying NAb level are applied because direct measurement of IFN-β antiviral activity is complicated. This study was aimed at standardization and validation of a reporter cell system for measuring anti-human IFN-β NAb titers, and evaluation data were obtained with samples from 33 patients with multiple sclerosis.
KEY WORDS: multiple sclerosis, immunogenicity, interferon-beta, neutralizing antibodies, titerDOI: 10.1134/S000629791611016X
Abbreviations: BTPs, biotherapeutic products; IFN-β, interferon-beta; LU, laboratory unit; MS, multiple sclerosis; NAbs, neutralizing antibodies; RF, rheumatoid factor.
Genetically engineered biological preparations, or biotherapeutic
products (BTPs), are proteins obtained using recombinant DNA
technology. BTPs consist of recombinant proteins and monoclonal
antibodies modified by genetic engineering [1, 2]. In contrast to lower molecular weight
pharmaceuticals, BTPs exhibit immunogenicity directed against both its
own molecules as well as cross-reacting body proteins [3]. In some cases, immunogenicity represents the main
advantage of BTPs. In particular, high immunogenicity of components
contained in recombinant vaccine determines its therapeutic efficacy
[4]. However, sometimes increased immunogenicity
hinders development of novel directions for use of BTPs. This problem
became especially urgent in connection with the expanding list of drugs
widely used in modern medicine. Differences in primary sequence and
posttranslational modifications distinguishing protein BTP and
endogenous protein are among multiple factors determining
immunogenicity. Moreover, route of administration, impurities, shelf
life, and formulation stability as well as genetic predisposition
responsible for individual immune response affect BTP immunogenicity
[5].
As early as 1993, human recombinant IFN-β was one of the first BTPs approved for medical use to treat multiple sclerosis (MS) [6]. Still, the exact mechanism of therapeutic action for IFN-β remains unclear, but its administration during MS lowers permeability of the blood–brain barrier, suppresses migration of immune cells, and modifies cytokine production and T-cell responses. Repeated subcutaneous administration of IFN-β induces a pronounced humoral immune response [7].
It is believed that high molecular weight aggregates formed during storage are the most important cause for developing augmented immune response against BTPs. Upon subcutaneous administration, protein aggregates exhibit marked adjuvant properties owing to a high density of repeated T- and B-epitopes and induction of secondary inflammation [8]. A direct correlation between development of protein aggregates, heightened immunogenicity, and lowered biological activity of BTPs was demonstrated in many studies [3, 8, 9]. Posttranslational glycosylation significantly influences drug solubility and pharmacokinetics as well as tendency to aggregate. It was shown that 98% of glycosylated IFN-β-1a molecules in solution are found in monomeric form, whereas 60% of non-glycosylated IFN-β-1b consists of bulky soluble 600-kDa aggregates [8]. Artificial deglycosylation of IFN-β-1a molecules increases their sensitivity to denaturation and reduces biological activity due to formation of insoluble aggregates linked by disulfide bonds [9]. A relationship between high immunogenicity and BTP protein aggregates was also found for recombinant growth factor, coagulation factor VIII, monoclonal antibodies, and IFN-α [3].
BTPs may trigger production of binding and neutralizing antibodies. A pool of binding antibodies appearing at early stages of IFN-β therapy gradually becomes replaced by neutralizing antibodies (NAbs) during ongoing immunization with BTP, which block domains in IFN-β responsible for its binding to specific cell receptors. This significantly reduces or completely inhibits therapeutic and biological activity of BTPs. Depending on BTP formulation, detection method, and time point, the prevalence of NAbs against IFN-β greatly varies and reaches 2-36% [8]. Significant NAb titers appear 9-18 months after the onset of therapy. As early as 1996, a negative effect exerted by antibodies on IFN-β therapeutic efficacy, now considered a proven fact, was already found during the first studies with Betaferon [10].
Suppression of IFN-β biological activity is considered as the main property of NAbs. A cytopathic effect inhibition assay is a classic approach for measuring interferon bioactivity, and concentration of 1 LU/ml IFN-β corresponds to the maximum dilution providing protection to 50% of cultured cells from virus cytodestructive effect. However, although still being a “golden standard”, this assay due to its complexity was not widely adopted into routine laboratory practice [11].
A reporter transgenic test-system based on HL-116 human fibrosarcoma cell line that was stably transfected with luciferase gene controlled by Interferon Stimulated Response Element (ISRE) is a promising approach for developing a bioassay for measuring anti-IFN-β NAbs. In particular, binding of IFN-β to a specific receptor triggers a signaling cascade resulting in transcription of the reporter gene followed by production of luciferase protein. Measuring its level allows indirect assessment of the level of IFN-β biological activity as well as NAb concentration [12, 13]. This approach was applied to develop an assay for measuring anti-IFN-β NAbs in MS patients.
MATERIALS AND METHODS
During the study, 33 MS patients treated with IFN-β-1a for ≥1 year at the St. Petersburg City Center for Multiple Sclerosis and Autoimmune Diseases were enrolled. The study protocol was approved by the Ethics Committee at City Clinical Hospital No. 31, St. Petersburg, Russia. All patients signed voluntary informed consent for participation in the study.
Blood sera collected from five patients suffering from other autoimmune diseases were used to validate and standardize methods used in the study: rheumatoid arthritis confirmed by diagnostic criteria with rheumatoid factor (RF) ranging from 60 to 2000 IU/ml, control specimens positive for antibodies against double-stranded DNA (certified value 1 : 320), smooth muscle (certified value 1 : 160), anti-nuclear factor (certified value 1 : 500), ten blood donors who did not receive IFN-β preparations or have any autoimmune diseases, two control goat serum samples containing high- (1 : 320) and low- (1 : 20) titer antibodies against human interferon, and negative goat serum sample (0 TRU).
HT-1080 human fibrosarcoma cell line (ATCC) clone HL-116 (this and control anti-IFN antibody samples are courtesy of Professor G. Giovannoni, London, Great Britain) stably transfected with ISRE-controlled luciferase gene was used to measure anti-IFN-β NAb titers [13]. Cell lines were grown in DMEM (Biolot, Russia) culture medium supplemented with 10% fetal bovine serum (Biolot), 1% antibiotic/antimycotic cocktail, and selective medium (hypoxanthine-aminopterin-thymidine medium). Cells at 80% confluent monolayer were seeded into 96-well plates for adherent cells (Thermo Scientific, USA) and further cultured for 24 h. Cell concentration was 4·104 cells/well.
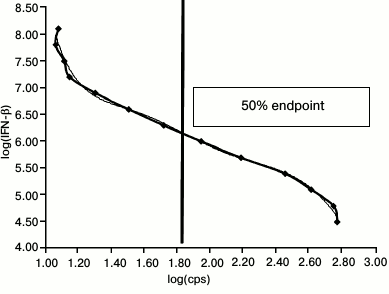
To calculate 1 LU/ml concentration, a standard 2-fold dilution curve for therapeutic IFN-β-1a (Avonex, Russia) preparation dissolved in culture medium within the range 200-0.05 IU/ml was plotted. It is known that 10 LU/ml IFN-β-1a (Avonex) corresponds to 20 IU/ml. Due to high IFN-β concentration, it was initially diluted to 1000 IU/ml (corresponding to 500 LU/ml), and then – to 200 IU/ml (corresponding to 100 LU/ml). After that, the cell line was incubated with IFN-β at each dilution for 5 h followed by adding substrate for luciferase (luciferin). Then, the cells were lysed to measure intensity of luminescent signal (shown as counts per second, cps) using a Steady Glo Luciferase Kit (Promega, USA) in an LM-01T thermostatted microplate luminometer (Immunotech, Czech Republic) and analyzed using LIANA software. A standard dilution curve was then plotted. Logarithmic intensity of luminescent signal (cps) is shown on the X-axis, whereas logarithmic IFN-β dilutions – on the Y-axis. Endpoint dilution equal to one laboratory unit (1 LU/ml) and corresponding to 50% luminescent signal obtained in the standard dilution curve was calculated by applying an equation for polynomial regression (Fig. 1).
Fig. 1. Standard IFN-β-1a 2-fold dilution curve ranging within 200-0.05 IU/ml. X-axis: logarithm of counts per second (log(cps)) for luminescent signal is plotted; Y-axis: logarithm of IFN-β dilution (log(IFN-β)). The vertical line denotes endpoint equal to 1 LU/ml.
To measure amount of anti-IFN-β NAbs, serum samples serially diluted at (1 : 20)-(1 : 2560) were incubated for 1 h with IFN-β-1a at concentration 10 LU/ml, which was calculated according to the standard dilution curve. After that, the cell line placed into a 96-well plate was incubated with a mixture containing serum sample and interferon for 5 h, followed by adding luciferin and transferring cell lysate into a luminometer plate to measure intensity of luminescent signal. All samples were analyzed in duplicates, and data are presented as average values.
To precisely calculate 1 LU/ml value and error correction, a standard IFN-β dilution curve ranging within 10-0.08 LU/ml was plotted in each experiment. A correction factor (N) was calculated by dividing dilution factor for IFN-β required to reach 1 LU/ml endpoint by dilution factor for IFN-β required to reach endpoint on correction curve. This allows correcting potential error in IFN-β dilutions and normalizing impact of random factors during cell culturing.
Endpoint titer was calculated by determining 1 LU/ml value on the serum dilution curve and IFN-β dilution curve.
A correction factor was then used to calculate NAb neutralizing titer according to the formula:
where T – corrected NAb titer, F – serum dilution reducing biological activity to 1 LU/ml, N – correction factor [14].
Reference anti-IFN-β NAb values described in the international recommendations were used in the study. Serum samples were assessed as follows: negative – anti-IFN-β NAb titer <20; low/intermediate titer – 20-100; high titer >100 [10]. The method used to measure anti-IFN-β NAbs was validated and standardized as follows: reproducibility, detection limits, and effect of irrelevant antibodies on IFN-β biological activity were determined. After that, 10 blood donors and 33 MS patients were screened for anti-IFN-β NAbs to further calculate precise antibody titer.
The data were statistically processed using GraphPad Prism 6.0 software. The relationship between two variables was examined using the Spearman rank correlation method. For all statistical methods, significance level was set at p< 0.05.
RESULTS
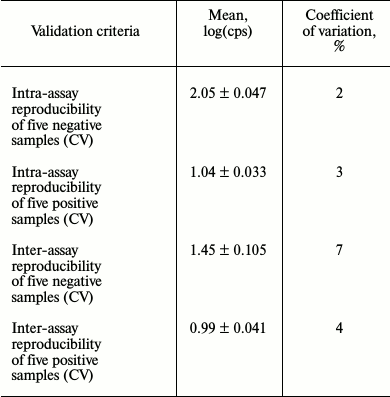
To check reproducibility, an assay for detecting NAbs against IFN-β preparations with HL-116 reporter cell line was validated (Table 1).
Table 1. Examination of test system
reproducibility
A series of experiments with positive control sample (NAb titer 1000) was carried out to assess analytical accuracy. The standard deviation was found to be 61.32 (Table 2).
Table 2. Number of repeated measurements of
control samples in one series
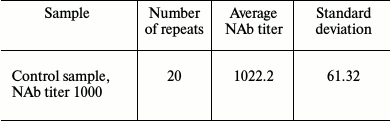
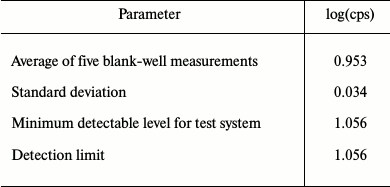
Five sequential measurements of FCS/IFN-free DMEM medium were performed to determine the minimum detection limit, which was found to be 1.056 (Table 3).
Table 3. Measurement of minimum detectable
level for test system
To investigate potential interference with other autoantibodies often developed in patients with autoimmune disorders, we examined serum samples from five patients with rheumatoid arthritis having high RF (60-2000 IU/ml) as well as serum samples containing antibodies against double-stranded DNA (certified value 1 : 320), smooth muscle (certified value 1 : 160), and antinuclear factor (certified value 1 : 500). We found that regardless of RF concentration as well as antinuclear antibodies, smooth muscle- and double-stranded DNA-specific antibodies, they had no impact on IFN-β biological activity.
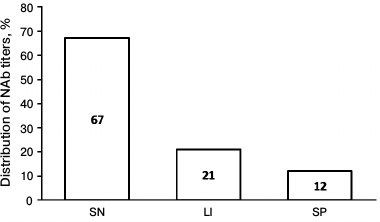
After the validation procedure, the assay was tested using samples from 33 MS patients treated with IFN-β-1a therapy for more than 1 year as well as from 10 volunteers having no anti-IFN-β NAbs. The screening revealed anti-IFN-β NAbs in 11 of 33 samples (33.3%). Moreover, clinically significant increase in NAb titer (>20) was found in 7 of 11 positive samples, whereas extremely high amount of NAbs was detected in 4 of 11 samples (Fig. 2).
Fig. 2. Diagram demonstrating distribution of anti-IFN-β NAb titer in examined patients. SN, seronegative patients, NAb titer <20; LI, patients with low/intermediate NAb titer, 20-100; SP, seropositive patients with extremely high NAb titer, >100.
DISCUSSION
BTP-based therapy is used in many fields of medicine, primarily in treatment of chronic diseases. In many cases, BTP therapy represents the only alternative to symptomatic treatment preventing early disability and mortality. Currently, high immunogenicity of proteinaceous pharmaceuticals and therapeutic activity inhibited by NAbs, which are synthesized against them, become more prominent in personalized medicine. Use of cell-based systems for detecting NAbs against BTPs such as IFN-β allows direct assessment of the impact of the antibody pool on pharmacological and biological activity of such expensive preparations. Reporter cell lines represent promising test systems for assessing biological activity of BTPs in vitro. Compared to standard cytopathic assays, use of genetically modified cell lines for assessing BTP immunogenicity allows direct estimation of the impact of antibody pool on pharmacological and biological activity of expensive preparations. Simple and quick cell culturing technique, reproducibility of biological responses, and no need to deal with viruses are among advantages of such bioassays, which make reporter cell systems promising test platforms for assessing BTP biological activity in vitro as well as detection of NAbs.
Theoretically, it is expected that high affinity antibodies completely neutralize a fixed amount of biomolecules, and residual activity, therefore, depends on initial concentration of input substance. However, experimental data demonstrate that proportional neutralization activity dominates among the majority of occurring anti-BTP antibodies, i.e. low-affinity antibodies neutralize only some fraction of the biomolecule activity. A 10-fold decrease of biomolecule activity, proportional to 10-to-1 LU/ml activity decrease, is considered as a neutralization unit. The method used in this study implies titration of antibody activity at fixed BTP concentration for determining NAb dilution, which results in reduction of biopreparation activity to 1 LU/ml. To calculate antibody neutralization titer, a correction coefficient for serum dilution was used that results in decrease in BTP activity to 1 LU/ml, and defined as (n – 1)/9, where n – initial BTP concentration in the system. Further details on calculating antibody neutralization titer can be found in an article by Kawade et al. [14].
Validation of the anti-IFN-β NAb detection procedure based on reporter HL-116 fibrosarcoma cell line revealed the major analytical parameters of this assay and proved its applicability in routine laboratory practice. By assessing analytical characteristics, this bioassay demonstrated high within-laboratory reproducibility when the same positive and negative samples were analyzed in the same laboratory after repeating the full procedure for sample preparation and conducting all measurements in different sets of samples. The coefficient of variation (CV%) did not exceed 15% cut-off value, which is critical to immunometric tests, and all validation data agree with international and domestic validation criteria [15].
By performing clinical testing of our validated test system, we found that NAbs were present in 33.3% patients, among which extremely high antibody titers were detected in 12% of patients, which agrees with the data from several randomized prospective, non-randomized prospective, and retrospective studies [16, 17]. The data obtained in the current study are consistent with the results from earlier clinical studies with IFN-β-1a preparations and others [18, 19]. For instance, PRISM and SPECTRIMS studies showed that after long-term IFN-β-1a therapy, NAbs were detected in 15-22% of patients [18].
During the current study, we were able to validate and standardize a method for detecting NAbs against IFN-β preparations that agrees with domestic and international recommendations. Being analyzed together with other immunological parameters, our approach can be used for laboratory monitoring of MS patients as well as for developing and improving novel domestic BTPs.
Acknowledgements
The study was performed with financial support by the Russian Science Foundation (project No. 16-15-00118).
REFERENCES
1.Rader, R. (2008) Redefining biopharmaceutical,
Nat. Biotechnol., 26, 743-751.
2.Baumann, A. (2006) Early development of therapeutic
biologics – pharmacokinetics, Curr. Drug Metab.,
7, 15-21.
3.Wadhwa, M., Knezevic, I., Kang, H. N., and Thorpe,
R. (2015) Immunogenicity assessment of biotherapeutic products: an
overview of assays and their utility, Biologicals, 43,
298-306.
4.WHO. Guidelines on clinical evaluation of vaccines:
regulatory expectations (2004) WHO Tech Rep., 924,
35-102.
5.Van Beers, M. M. C., and Bardor, M. (2012)
Minimizing immunogenicity of biopharmaceuticals by controlling critical
quality attributes of proteins, Biotechnol. J., 7,
1473-1484.
6.Nazarov, V. D., Lapin, S. V., Surkova, E. A.,
Evdoshenko, E. P., Makshakov, G. S., and Totolian, A. A. (2015)
Diagnostic and prognostic significance of intrathecal synthesis of
immunoglobulin free light chains in multiple sclerosis, Med.
Immunol. (Moscow), 17, 235-244.
7.Nikfar, S., Rahimi, R., and Abdollahi, M. (2010) A
meta-analysis of the efficacy and tolerability of interferon-β in
multiple sclerosis, overall and by drug and disease type, Clin.
Ther., 32, 1871-1888.
8.Govindappa, K., Sathish, J., Park, K., Kirkham, J.,
and Pirmohamed, M. (2015) Development of interferon beta-neutralizing
antibodies in multiple sclerosis – a systematic review and
meta-analysis, Eur. J. Clin. Pharmacol., 71,
1287-1298.
9.Van Beers, M. M., Jiskoot, W., and Schellekens, H.
(2010) On the role of aggregates in the immunogenicity of recombinant
human interferon beta in patients with multiple sclerosis, J.
Interf. Cytokine Res., 30, 767-775.
10.Polman, C. H., Bertolotto, A., Deisenhammer, F.,
Giovannoni, G., Hartung, H. P., Hemmer, B., Killestein, J., McFarland,
H. F., Oger, J., Pachner, A. R., Petkau, J., Reder, A. T., Reingold, S.
C., Schellekens, H., and Sorensen, P. S. (2010) Recommendations for
clinical use of data on neutralizing antibodies to interferon-beta
therapy in multiple sclerosis, Lancet Neurol., 9,
740-750.
11.WHO Expert Committee on Biological
Standardization. Thirty-fifth report (1985) World Health Organ.
Tech. Rep. Ser., 725, 1-140.
12.Deisenhammer, F. (2014) Interferon-beta:
neutralizing antibodies, binding antibodies, pharmacokinetics and
pharmacodynamics, and clinical outcomes, J. Interferon Cytokine
Res., 34, 938-945.
13.Lam, R., Farrell, R., Aziz, T., Gibbs, E.,
Giovannoni, G., Grossberg, S., and Oger, J. (2008) Validating
parameters of a luciferase reporter gene assay to measure neutralizing
antibodies to IFNβ in multiple sclerosis patients, J. Immunol.
Methods, 336, 113-118.
14.Kawade, Y., Finter, N., and Grossberg, S. E.
(2003) Neutralization of the biological activity of cytokines and other
protein effectors by antibody: theoretical formulation of antibody
titration curves in relation to antibody affinity, J. Immunol.
Methods, 278, 127-144.
15.Meager, A., Dolman, C., Dilger, P., Bird, C.,
Giovannoni, G., Schellekens, H., Thorpe, R., and Wadhwa, M. (2011) An
assessment of biological potency and molecular characteristics of
different innovator and noninnovator interferon-beta products, J.
Interferon Cytokine Res., 31, 383-392.
16.Gneiss, C., Tripp, P., Reichartseder, F., Egg,
R., Ehling, R., Lutterotti, A., Khalil, M., Kuenz, B., Mayringer, I.,
Reindl, M., Berger, T., and Deisenhammer, F. (2006) Differing
immunogenic potentials of interferon beta preparations in multiple
sclerosis patients, Mult. Scler., 12, 731-737.
17.Ross, C., Clemmesen, K. M., Sorensen, P. S.,
Koch-Henriksen, N., and Bendtzen, K. (2006) Measuring and evaluating
interferon β-induced antibodies in patients with multiple
sclerosis, Mult. Scler., 12, 39-46.
18.Manfredonia, F., Pasquali, L., Dardano, A.,
Iudice, A., Murri, L., and Monzani, F. (2008) Review of the clinical
evidence for interferon β 1a (Rebif) in the treatment of multiple
sclerosis, Neuropsychiatr. Dis. Treat., 4, 321-336.
19.The IFNB Multiple Sclerosis Study Group (1993)
Interferon beta-1b is effective in relapsing-remitting multiple
sclerosis. I. Clinical results of a multicenter, randomized,
double-blind, placebo-controlled trial, Neurology, 43,
655-661.