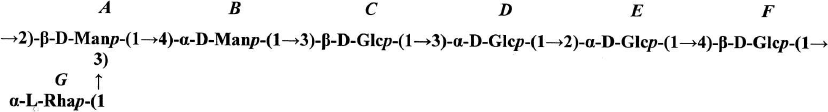Rhamnose-Containing Cell Wall Glycopolymers from Rathayibacter toxicus VKM Ac-1600 and “Rathayibacter tanaceti” VKM Ac-2596
A. S. Shashkov1, E. M. Tul’skaya2,a*, A. S. Dmitrenok1, G. M. Streshinskaya2, N. V. Potekhina2, S. N. Senchenkova1, N. F. Piskunkova2, L. V. Dorofeeva3, and L. I. Evtushenko3
1Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, 119991 Moscow, Russia2Lomonosov Moscow State University, Faculty of Biology, 119991 Moscow, Russia
3All-Russian Collection of Microorganisms (VKM), Skryabin Institute of Biochemistry and Physiology of Microorganisms, Russian Academy of Sciences, 142290 Pushchino, Moscow Region, Russia
* To whom correspondence should be addressed.
Received December 26, 2017; Revision received January 31, 2018
Structures of the cell wall glycopolymers from two representatives of the genus Rathayibacter were investigated using chemical, NMR spectroscopy, and optical methods. The R. toxicus VKM Ac-1600 strain contains two neutral glycopolymers – a linear rhamnomannan →2)-α-D-Rhap-(1→3)-α-D-Manp-(1→ and a branched polysaccharide containing in the repeating unit the residues of D-Manp, D-Glcp, and L-Rhap in the ratios of 2 : 4 : 1, respectively (the structure is presented in the text). The “Rathayibacter tanaceti” VKM Ac-2596 contains a rhamnomannan that is different from the above-described one by localization of glycosidic bonds on the residues of α-Rhap and α-Manp, i.e. →3)-α-D-Rhap (1→2)-α-D-Manp-(1→. The structures of all identified glycopolymers are described for the first time in actinobacteria. The data obtained make it possible to characterize representatives of the studied actinobacteria more fully and can be used to differentiate Rathayibacter species at the phenotype level.
KEY WORDS: Rathayibacter, cell wall, rhamnomannan, neutral glycopolymers, D-rhamnose, NMR spectroscopyDOI: 10.1134/S0006297918060093
Abbreviations: δC and δH, chemical shifts of 13C and 1H atoms, respectively; COSY, correlation spectroscopy; HMBC, heteronuclear multiple-bond correlation; HSQC, heteronuclear single quantum coherence; J, spin-spin interaction constant; ROE, one-dimensional rotating frame Overhauser effect spectroscopy; ROESY, two-dimensional rotating frame Overhauser effect spectroscopy; TOCSY, total correlation spectroscopy; TSP, sodium salt of 3-(trimethylsilyl)-3,3,2,2-tetradeuteropropionic acid; VKM, All-Russian Collection of Microorganisms.
Actinobacteria of the Rathayibacter genus (family
Microbacteriaceae, order Micrococcales) are aerobic, Gram-positive
irregular rods with a B2γ-type peptidoglycan and the prevalent
menaquinone MK-10 of the respiration chain [1, 2]. The genus now includes six species with validly
published names ([1, 3, 4], http://www.bacterio.net/rathayibacter.html).
There are also data about other species of the genus that are isolated
but not yet described validly [5-7]. Rathayibacter rathayi, R. iranicus,
R. tritici and R. toxicus are known as phytopathogens
causing gummosis and growth delay of cereals (the Poaceae family) [2]. Moreover, R. toxicus produces a
tunicamycin-like toxin responsible for neurotoxicosis and death of
herbivore animals [3]. Under natural conditions,
phytopathogenic species of Rathayibacter are transferred onto
host plants by their vectors – gall producing nematodes of the
Anguina genus [2, 7, 8].
Studies on the cell wall polymers from actinobacteria of the Rathayibacter genus are interesting, in particular in connection with specific features of the molecular composition of cell walls in microorganisms of various taxa and for understanding mechanisms of interactions of the bacteria with plants and nematode vectors. Studies of the cell wall glycopolymers enlarge the concepts about the diversity of natural polymers and biosynthetic potential of microorganisms. In the cell walls of representatives of some genera of coryneform actinobacteria from the order Micrococcales (Arthrobacter, Agromyces, Promicromonospora), glycopolymers of various classes and structures not previously described have been identified [9-13]. The cell wall glycopolymers of representatives of the genus Rathayibacter have not yet been studied.
In this work, we presented results of studies on the cell wall glycopolymers of two strains from the Rathayibacter genus, i.e. R. toxicus VKM Ac-1600 initially isolated from the seeds of Phalaris minor, family Poaceae, on the region infected with the nematode Anguina funesta [1], and “Rathayibacter tanaceti” VKM Ac-2596 not yet validly described as a new species of Rathayibacter genus isolated from the leaf of tansy (Tanacetum vulgare, family Asteraceae) infected with the leaf nematode Aphelenchoides fragariae [5].
MATERIALS AND METHODS
In this work, strains from the All-Russian Collection of Microorganisms (VKM) at the Skryabin Institute of Biochemistry and Physiology of Microorganisms, Russian Academy of Sciences (www.vkm.ru) were used: R. toxicus VKM Ac-1600 (= ICMP 6309) and “Rathayibacter tanaceti” VKM Ac-2596 [5].
The cultures were grown under aerobic conditions at 28°C in flasks on a shaker until mid-log phase using peptone–yeast medium [14].
Cell walls were obtained by differential centrifugation after the cells were treated using a UP100H ultrasound disintegrator (Hielscher, Germany) for (3-5) × 10 min in water at 4°C, treated subsequently with 2% SDS at 100°C for 5 min, and lyophilized.
Glycopolymers were isolated from the cell walls (~500 mg) as described in the work of Potekhina et al. [14]. Successive extractions were performed using 10% TCA (3 × 24 h at 4°C, the extracts were combined) and then 5% TCA (20 min at 90°C). The yield of glycopolymer preparations obtained from the cool and hot extractions was, respectively, 2.3 and 4.3% for strain VKM Ac-1600 and 2.6 and 14.6% for strain VKM Ac-2596. Later, the glycopolymer preparations manifesting a complete identity in the preliminary chemical and NMR spectroscopic studies were combined and denominated as preparation Ac-1600 and preparation Ac-2596, respectively.
Acidic hydrolysis of the cell wall and glycopolymer preparations were performed with 2 M HCl for 3 h at 100°C.
Descending chromatography and electrophoresis were performed on paper (Filtrak FN-3; Germany) using different systems of solvents [14]. Phosphate-containing compounds were detected with the Isherwood reagent; compounds containing amino group were detected with ninhydrin; polyols and monosaccharides with 5% AgNO3; reducing monosaccharides with aniline phthalate [14].
Polymers in preparation Ac-1600 were separated by gel chromatography on a column (37.5 × 2.6 cm) with Sephadex G-50 Superfine (Amersham Biosciences, Sweden) [15].
Absolute D-configurations of rhamnose and mannose were established by determination of the specific rotation value of aqueous solutions of polymers I and III on a P-2000 digital polarimeter (JASCO Corporation, Japan) using the Klyne rule [16] and based on literature data on the specific rotation of monosaccharides [17, 18]. The absolute configuration of monosaccharide residues in polymer II was determined by glycosylation effects in the 13C-NMR spectra according to the patterns described earlier [19] and assuming that glucose belongs to the D-series.
NMR spectra were recorded using a Bruker AV600 installation (Bruker, Germany) for solutions in 99.96% D2O at temperatures providing the minimal overlapping of the deuterium-loaded water signal with signals of the polymers. As an internal etalon, TSP was used (δH 0.0 ppm, δC —1.6 ppm). Two-dimensional spectra were taken and recorded using standard methods of the Bruker firm. The spin-lock time in the TOCSY and HSQC-TOCSY experiments were 100 and 60 ms, respectively. The mixing time in the ROESY experiment was 150 ms. Experiments HMBC were optimized for constants of spin–spin interaction JH,C 8 Hz.
RESULTS AND DISCUSSION
Structures of glycopolymers from two strains of the Rathayibacter genus have been studied by chemical, NMR spectroscopic, and optical methods. One-dimensional 1H- and 13C-NMR spectra of all preparations studied were recorded. For identification of the one-dimensional spectra and determination of the glycopolymer structures, two-dimensional homonuclear 1H,1H-COSY, TOCSY, ROESY spectra were recorded and identified, as well as heteronuclear 1H,13C-HSQC, HSQC-TOCSY, and HMBC spectra.
Rathayibacter toxicus VKM Ac-1600. Hydrolysates of the cell walls were found to contain mineral phosphate, glucose, mannose, rhamnose, galactose, and xylose.
Among products of acidic hydrolysis of preparation Ac-1600, glucose, mannose and rhamnose were identified, which could be constituents of glycopolymers of the cell wall of this actinobacterium.
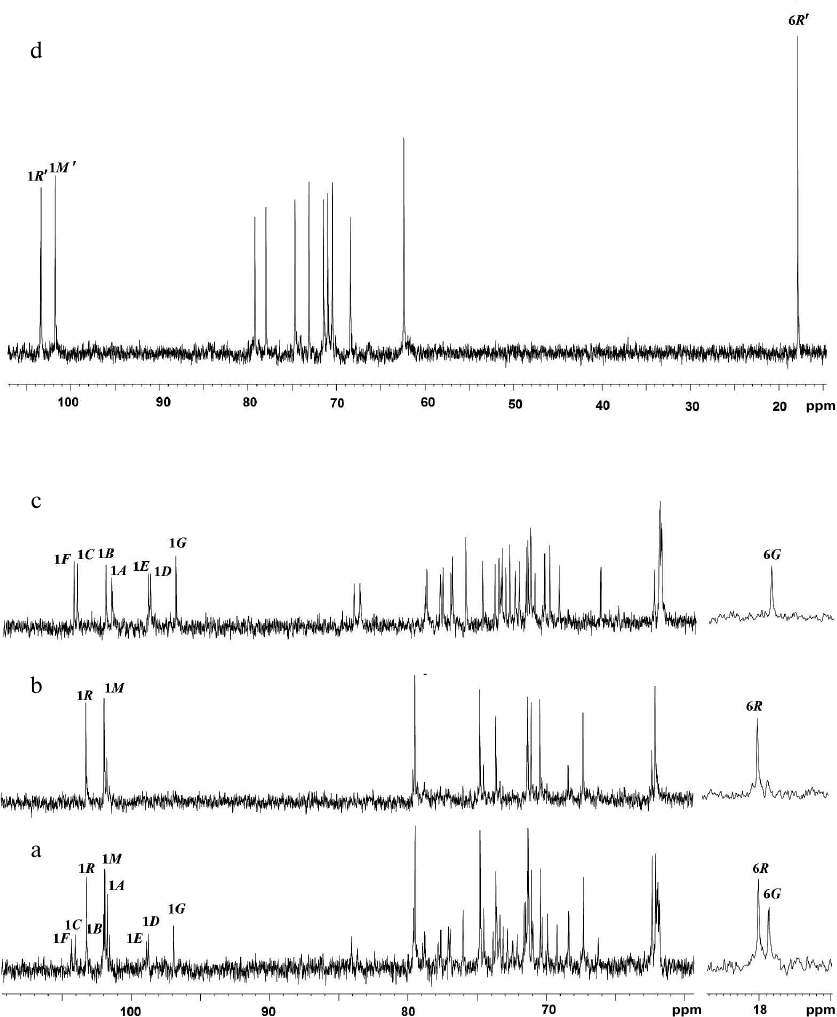
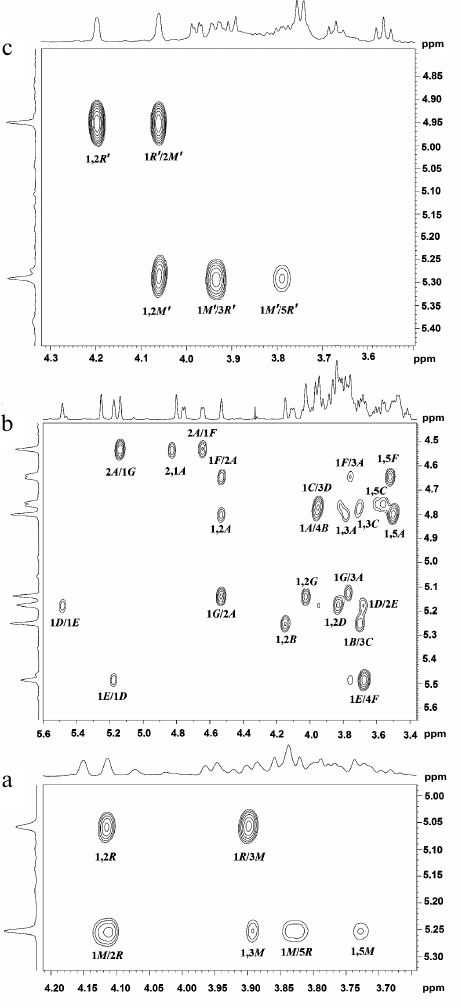
In the anomeric region of the 13C-NMR spectrum (table and Fig. 1a) of preparation Ac-1600, two sets of signals with different intensity were detected: signals at δC 103.2 and 101.9 ppm and signals with lower intensity in the region of 97-104 ppm. The spectrum did not contain signals of carboxyl groups and appeared as belonging to a mixture of two neutral polymers. Therefore, preparation Ac-1600 was subjected to gel chromatography on a column with Sephadex G-50. As a result, two fractions were obtained. The fraction with the lower molecular mass (Fig. 1b) belonged to the regular polysaccharide having two monosaccharide residues in the repeating unit (polymer I). The fraction with the high molecular mass (Fig. 1c) represented a regular polysaccharide with a seven-member repeating unit (polymer II). In the anomeric region of the 1H-NMR spectra of the two polymers presented in the upper part of the 1H,13C-HSQC spectra (Fig. 2, a and b), there are well-resolved signals: two for polymer I (Fig. 2a) and seven for polymer II (Fig. 2b).
Chemical shifts in the 13C- and 1H-NMR spectra of
glycopolymers from the cell walls of R. toxicus VKM Ac-1600 and
“Rathayibacter tanaceti” VKM Ac-2596
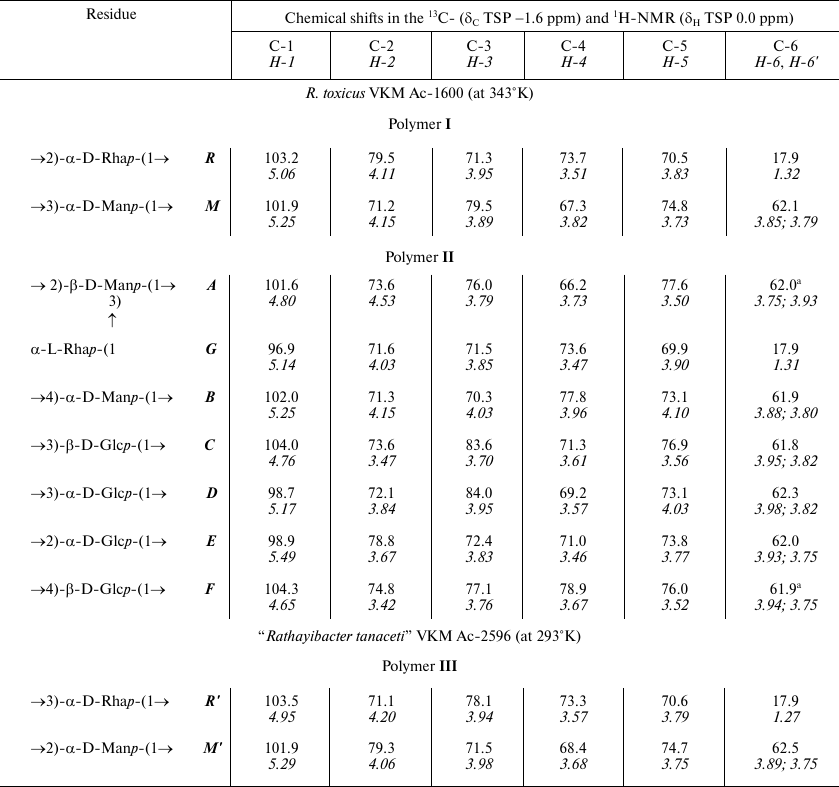
a Arbitrary assignment.
Fig. 1. 13C-NMR spectra of glycopolymers of the cell walls from R. toxicus VKM Ac-1600 and “Rathayibacter tanaceti” VKM Ac-2596. a) Preparation Ac-1600; b) polymer I; c) polymer II; d) polymer III. Arabic numerals indicate numbers of carbon atoms in the residues shown by capital Latin letters in accordance with the table.
Fig. 2. Parts of two-dimensional 1H,13C-HSQC spectra of glycopolymers from the cell walls of R. toxicus VKM Ac-1600 and “Rathayibacter tanaceti” VKM Ac-2596. a) Polymer I; b) polymer II; c) polymer III. The corresponding parts of the one-dimensional 1H and 13C NMR spectra are presented above and to the left of the two-dimensional spectrum, respectively. Arabic numerals indicate numbers of carbon atoms in the residues shown by capital Latin letters in accordance with the table.
The 1H,1H-COSY and TOCSY spectra of polymer I revealed residues of α-rhamnopyranose (α-Rhap) and α-mannopyranose (α-Manp) as monosaccharide constituents of the repeating unit.
The shift in the 1H,13C-HSQC spectrum (table and Fig. 2a, to the left) of carbon atom signals in comparison with such signals of the initial pyranoses indicate the replacement of α-Rhap residues into position 2 (the chemical shift C-2 at 79.5 ppm) and of α-Manp residues into position 3 (the chemical shift C-3 at 79.5 ppm).
The linearity of the polymeric chain was proved by analyzing the 1H,1H-ROESY spectrum (Fig. 3a), where correlation peaks were observed between residues H-1(R)/H-3(M) and H-1(M)/H-2(R). Calculations of the glycosylation effects for C-1 α-Rhap (+8.0 ppm) clearly showed the identity of absolute configurations of α-Rhap and α-Manp according to the pattern detected in a work by Lemieux and Levine [18]. The absolute D-configuration of the two residues was determined based on the Klyne rule [16] and on the specific rotation of polymer I [α]D26° +74.0 (c 0.68), methyl-α-D-rhamnopyranoside [17], and methyl-α-D-mannopyranoside [18]. The structure of the repeating unit of polymer I can be presented as follows:
Fig. 3. Parts of two-dimensional 1H,1H-ROESY spectra of glycopolymers of the cell walls of R. toxicus VKM Ac-1600 and “Rathayibacter tanaceti” VKM Ac-2596. a) Polymer I; b) polymer II; c) polymer III. The corresponding parts of the 1H-NMR spectra are shown along the axes. Arabic numerals indicate numbers of carbon atoms in the residues shown by capital Latin letters in accordance with the table.
In polymer II, two-dimensional 1H,1H-COSY and TOCSY spectra, similarly to the one-dimensional 1H-TOCSY spectrum (Fig. S1, see Supplement to this paper on the site of the journal http://protein.bio.msu.ru/biokhimiya and Springer site Link.springer.com) as the constituents of the repeating unit the following monosaccharide residues were detected: of two β-glucopyranoses (β-Glcp), two α-glucopyranoses (α-Glcp), two α-mannopyranoses (α-Manp), and of α-rhamnopyranose (α-Rhap).
The shift in the 1H,13C-HSQC spectrum (Fig. 2b, to the left) of carbon atom signals in comparison with the signals of the initial pyranoses indicates the replacement of α-Manp residues at the C-2 and C-3 positions (table, see the structure below), and the other at position 4; residues β-Glcp are replaced at positions C-3 or C-4, whereas residues α-Glcp – at positions C-2 or C-3.
The residue α-Rhap was identified as terminal because its chemical shifts at C-2, C-3, and C-4 coincided with the shifts of the corresponding atoms of methyl-α-rhamnopyranoside [19].
The sequence of residues in the polymer (table, see the structure below) was established using one-dimensional 1H-ROE (Fig. S2, see the Supplement) and two-dimensional (Fig. 3b) 1H,1H-ROESY spectra in which correlation peaks were observed between protons H-1(A)/H-4(B); H-1(B)/H-3(C); H-1(C)/H-3(D); H-1(D)/H-1(E); H-1(D)/H-2(E); H-1(E)/H-4(F); H-1(F)/H-2(A), and H-1(G)/H-2,3(A). The sequence of residues in polymer II was confirmed by the presence of the correlation peaks H-1(A)/C-4(B); H-1(B)/C-3(C); H-1(C)/C-3(D); H-1(D)/C-2(E); H-1(E)/C-4(F); H-1(F)/C-2(A), and H-1(G)/C-3(A) in the 1H,13C-HMBC spectrum (Fig. 4a).
Fig. 4. Two-dimensional 1H,13C-HMBC spectra of glycopolymers from the cell walls of R. toxicus VKM Ac-1600 and “Rathayibacter tanaceti” VKM Ac-2596. a) Polymer II; b) polymer III. The corresponding parts of the one-dimensional 1H- and 13C-NMR spectra are presented above and to the left of the two-dimensional spectrum, respectively. Arabic numerals indicate the numbers of carbon atoms in the residues shown by capital Latin letters in accordance with the table.
The absolute configurations of monosaccharide residues were determined based on the earlier described pattern [19] and assuming that glucose should belong to the D-series. A small positive (+0.4 ppm) β-effect of the replacement was observed for C-4 β-Glcp (residue C) as a result of glycosylation with a monosaccharide possessing the α-configuration of the anomeric center (α-Manp, residue B). The value +0.4 ppm is characteristic for the same absolute configuration (D) of residues B and C [18]. A small negative effect (—1.2 ppm) for C-3 of residue B as a result of its glycosylation at C-4 with pyranose having the β-configuration of the anomeric center also corresponds to the same (D) absolute configuration of the B and C residues. A very small α-effect of glycosylation (+1.7) for C-1 of α-Rhap (residue G) clearly indicated the different (L and D) absolute configuration of the G and A residues, respectively. For the identical absolute configuration, the effect of glycosylation would be much higher by the module (~ +8.0 ppm), as in the case of polymer I (see above). The structure of the repeating unit of polymer II is presented on Fig. 5.
Fig. 5. The structure of the repeating unit of polymer II.
“Rathayibacter tanaceti” VKM Ac-2596. Hydrolysates of the cell walls contained mineral phosphate, mannose, and rhamnose, as well as traces of galactose, glucose, and xylose.
Among products of the acidic hydrolysis of preparation Ac-2596, rhamnose and mannose were detected, which could be constituents of the cell wall glycopolymers of this actinobacterium.
The 13C-NMR spectrum (table and Fig. 1d) was typical for a regular polysaccharide with disaccharide repeating unit. It contained two signals in the region of the resonance of anomeric carbon atoms at δC 101.9 and 103.5 ppm, nine signals in the region of -CH-O- at δC 60-80 ppm, and a signal at δC 17.9 ppm. The 1H-NMR spectrum (Fig. 2c, above) had two signals in the anomeric region at δH 4.95 and 5.29 ppm with small values of constant of spin–spin interaction, one strong-field doublet (3H, δH 1.27 ppm, J 6 Hz), and signals in the region δC 3.55-4.2 ppm. Moreover, in the 1H-NMR spectrum very small signals of carbohydrate admixtures were observed. The 1H,1H-COSY and TOCSY spectra revealed residues of α-rhamnopyranose (α-Rhap, residue R′) and α-mannopyranose (α-Manp, residue M′) as constituents of the polysaccharide repeating unit. Thus, the polymer from preparation Ac-2596 has composition similar to that of polymer I from preparation Ac-1600. However, the 1H,13C-HSQC spectrum (Fig. 2c) shows another type of replacement in residues of polymer III, namely, at the C-3 position for α-Rhap and at the C-2 position for α-Manp. The 1H,1H-ROESY spectrum (Fig. 3c) showed correlation peaks between protons H-1 (R′)/H-2 (M′) and H-1 (M′)/H-3 (R′), demonstrating the linear type of polymeric chain.
The structure of the polysaccharide repeating unit was confirmed by the 1H,13C-HMBC spectrum (Fig. 4b), which had correlation peaks H-1 (R′)/C-2 (M′) and H-1 (M′)/C-2 (R′). The absolute D-configuration for both residues was determined by the specific rotation value [α]D26° +68.0 (c 1.5).
The structure of the polymer III repeating unit can be presented as follows:
The present work describes the composition and structure of glycopolymers from the cell walls of two strains of the genus Rathayibacter: R. toxicus VKM Ac-1600 and “Rathayibacter tanaceti” VKM Ac-2596, which were obtained from different habitats and possessed different nematode vectors. In both strains, phosphate-free rhamnose-containing glycopolymers were found.
Both microorganisms contain in their cell walls linear rhamnomannans characterized by the localization of glycosidic bonds on α-Rhap and α-Manp residues:
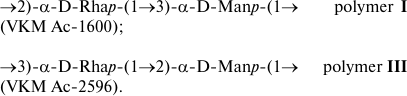
Rhamnomannans (with L-Rha) are described as constituents of peptide–rhamnomannans from the cell wall of the pathogenic yeast Sporothrix schenckii [20] and of glycoproteins from the S-layer of two strains of the Gram-positive bacterium Thermoanaerobacter thermohydrosulfuricus L 110-69 (=DSM 568) and L 111-69 [21], as well as in the structure of O-polysaccharides of some Gram-negative bacteria (with L- and D-Rha) (http://csdb.glycoscience.ru/).
Rhamnomannans of the studied Rathayibacter strains are characterized by the presence of residues of D-rhamnose, which is a rare constituent of the cell wall polymers of Gram-positive bacteria. For example, D-Rha was found in glycans of the S-layer of Aneurinibacillus thermoaerophilus [22-24], in a polysaccharide from the cell wall of Renibacterium salmoninarum, which is the agent of kidney disease in Salmonidae [25]. As a constituent of O-polysaccharides of Gram-negative bacteria, D-Rha is widely distributed along with L-Rha (http://csdb.glycoscience.ru/).
Thus, this is the first finding of rhamnomannans as glycopolymers of the cell walls in Gram-positive bacteria.
The cell wall of R. toxicus VKM Ac-1600 contains also a neutral polysaccharide, the polymer II possessing D-Man and D-Glc residues, as well as L-Rha in ratios 2 : 4 : 1 in the repeating unit. Its structure in the cell walls of actinobacteria is also described for the first time.
The presence of rhamnose as a constituent of the cell wall glycopolymers is thought to be associated with the pathogenicity and/or adhesive properties of bacteria and their ability to colonize plants [7, 26, 27]. It can be assumed that these properties are also inherent in the rhamnose-containing polysaccharides of the Rathayibacter genus representatives, on considering the biology and ecology of these bacteria [2, 3, 5, 7].
The present work also indicates that the studied representatives of different species of the Rathayibacter genus [5, 28] are different in the structure of the cell wall glycopolymers.
Thus, on the example of the studied strains, it is established that cell wall glycopolymers can be used for differentiation of species on the phenotype level. Moreover, the findings enlarge the concepts about the diversity of natural glycopolymers and biosynthetic potential of microorganisms.
Acknowledgments
This work was supported by the Russian Science Foundation (project No. 14-50-00126) and the Russian Foundation for Basic Research (project No. 13-04-00447).
REFERENCES
1.Zgurskaya, H. I., Evtushenko, L. I., Akimov, V. N.,
and Kalakoutskii, L. V. (1993) Rathayibacter gen, nov.,
including the species Rathayibacter rathayi comb. nov.,
Rathayibacter tritici comb. nov., Rathayibacter iranicus
comb., nov., and six strains from annual grasses, Int. J. Sys.
Bacteriol., 43, 143-149.
2.Evtushenko, L. I., and Dorofeeva, L. V. (2012)
Genus XXII. Rathayibacter, in Bergey’s Manual of
Systematic Bacteriology (Whitman, W., Goodfellow, M., Kampfer, P.,
Busse, H. J., Trujillo, M., Ludwig, W., Suzuki, K., and Parte, A.,
eds.) Vol. 5, Springer, New-York, pp. 953-964.
3.Riley, I. T., and Ophel, K. M. (1992)
Clavibacter toxicus sp. nov. the bacterium responsible for
annual ryegrass toxicity in Australia, Int. J. Syst. Bacteriol.,
42, 64-68.
4.Dorofeeva, L. V., Evtushenko, L. I., Krausova, V.
I., Karpov, A. V., Subbotin, S. A., and Tiedje, J. M. (2002)
Rathayibacter caricis sp. nov. and Rathayibacter festucae
sp. nov., isolated from the phyllosphere of Carex sp. and the
leaf gall induced by the nematode Anguina graminis on Festuca
rubra L., respectively, Int. J. Syst. Evol. Microbiol.,
52, 1917-1923.
5.Vasilenko, O. V., Starodumova, I. P., Tarlachkov,
S. V., Dorofeeva, L. V., Avtukh, A. N., and Evtushenko, L. I. (2016)
Draft genome sequence of “Rathayibacter tanaceti”
strain VKM Ac-2596 isolated from Tanacetum vulgare infested by a
foliar nematode, Genome Announc., 4, e00512-16.
6.Starodumova, I. P., Tarlachkov, S. V.,
Prisyazhnaya, N. V., Dorofeeva, L. V., Ariskina, E. V., Chizhov, V. N.,
Subbotin, S. A., Evtushenko, L. I., and Vasilenko, O. V. (2017) Draft
genome sequence of Rathayibacter sp. strain VKM Ac-2630 isolated
from leaf gall induced by the knapweed nematode Mesoanguina
picridis on Acroptilon repens, Genome Announc.,
5, e00650-17.
7.Murray, T. D., Schroeder, B. K., Schneider, W. L.,
Luster, D. G., Sechler, A., Rogers, E. E., and Subbotin, S. A. (2017)
Rathayibacter toxicus, other Rathayibacter species
inducing bacterial head blight of grasses, and the potential for
livestock poisonings, Phytopathology, 107, 804-815.
8.Riley, I. T., and McKay, A. C. (1990) Specificity
of the adhesion of some plant pathogenic microorganisms to the cuticle
of nematodes in the genus Anguina (Nematoda: Anguinidae),
Nematologica, 36, 90-103.
9.Gnilozub, V. A., Streshinskaya, G. M., Naumova, I.
B., Evtushenko, L. I., and Shashkov, A. S. (1994) 1,5-Poly(ribitol
phosphate) with tetrasaccharide substituent in the cell wall of
Agromyces fucosus ssp. hippuratus, Biochemistry
(Moscow), 59, 1419-1424.
10.Shashkov, A. S., Streshinskaya, G. M., Gnilozub,
V. A., Evtushenko, L. I., and Naumova, I. B. (1995) Poly(arabitol
phosphate) teichoic acid in the cell wall of Agromyces cerinus
subsp. cerinus VKM Ac-1340T, FEBS Lett.,
371, 163-166.
11.Shashkov, A. S., Potekhina, N. V., Kachala, V.
V., Senchenkova, S. N., Dorofeeva, L. V., and Evtushenko, L. I. (2012)
A novel galactofuranan from the cell wall of Arthrobacter sp.
VKM Ac-2576, Carbohydr. Res., 352, 215-218.
12.Potekhina, N. V., Shashkov, A. S., Senchenkova,
S. N., Dorofeeva, L. V., and Evtushenko, L. I. (2012) Structure of
hexasaccharide 1-phosphate polymer from Arthrobacter uratoxydans
VKM Ac-1979T cell wall, Biochemistry (Moscow),
77, 1294-1302.
13.Dmitrenok, A. S., Streshinskaya, G. M.,
Tul’skaya, E. M., Potekhina, N. V., Senchenkova, S. N., Shashkov,
A. S., Bilan, M. I., Starodumova, I. P., Bueva, O. V., and Evtushenko,
L. I. (2017) Pyruvylated cell wall glycopolymers of
Promicromonospora citrea VKM Ac-665T and
Promicromonospora sp. VKM Ac-1028, Carbohydr. Res.,
449, 134-142.
14.Potekhina, N. V., Streshinskaya, G. M.,
Tul’skaya, E. M., and Shashkov, A. S. (2011) Methods in
Microbiology (Rainey, F. A., and Oren, A., eds.) Vol. 38, Chap. 6,
Academic Press, Elsevier, pp. 132-164.
15.Shashkov, A. S., Streshinskaya, G. M., Kozlova,
Y. I., Tul’skaya, E. M., Senchenkova, S. N., Arbatskii, N. P.,
and Evtushenko, L. I. (2012) Teichulosonic acid, an anionic polymer of
a new class from the cell wall of Actinoplanes utahensis VKM
Ac-674T, Biochemistry (Moscow), 77,
633-640.
16.Klyne, W. (1950) The configuration of the
anomeric carbon atoms in some cardiac glycosides, Biochem. J.,
47, XLI-XLII.
17.Haskins, W. T., Hann, R. M., and Hudson, C. S.
(1946) The preparation of D-rhamnose from methyl
alpha-D-mannopyranoside, J. Am. Chem. Soc., 68,
628-632.
18.Lemieux, R. U., and Levine, S. (1962) The
products of the Prevost reaction on D-glucal triacetate, Can. J.
Chem., 40, 1926-1932.
19.Shashkov, A. S., Lipkind, G. M., Knirel, Y. A.,
and Kochetkov, N. K. (1988) Stereometrical factors determining the
effects of glycosylation on the 13C chemical shifts in
carbohydrates, Magn. Reson. Chem., 26, 735-747.
20.Lloyd, K. O., and Bitoon, M. A. (1971) Isolation
and purification of a peptido-rhamnomannan from the yeast form of
Sporothrix schenckii. Structural and immunochemical studies,
J. Immunol., 107, 663-671.
21.Messner, P., Egelseer, E. M., Sleytr, U. B., and
Schaffer, C. (2009) Microbial Glycobiology Structures,
Relevance and Applications (Moran, A. P., Holst, O., and von
Itzstein M., eds.) Pt. 7, Academic Press, Elsevier, pp. 109-128.
22.Kosma, P., Neuninger, C., Christian, R., Schulz,
G., and Messner, P. (1995) Glycan structure of the S-layer glycoprotein
of Bacillus sp. L420-91, Glycoconj. J., 12,
99-107.
23.Schaffer, C., Muller, N., Christian, R.,
Graninger, M., Wugeditsch, T., Scheberl, A., and Messner, P. (1999)
Complete glycan structure of the S-layer glycoprotein of
Aneurinibacillus thermoaerophilus GS4-97, Glycobiology,
9, 407-414.
24.Kneidinger, B., Graninger, M., Adam, G.,
Puchberger, M., Kosma, P., Zayni, S., and Messner, P. (2001)
Identification of two GDP-6-deoxy-D-lyxo-4-hexulose reductases
synthesizing GDP-D-rhamnose in Aneurinibacillus thermoaerophilus
L420-91, J. Biol. Chem., 276, 5577-5583.
25.Sorum, U., Robertsen, B., and Kenne, L. (1998)
Structural studies of the major polysaccharide in the cell wall of
Renibacterium salmoninarum, Carbohydr. Res., 306,
305-314.
26.Riley, I. T., Allen, J. G., and Barbetti, M. J.
(2014) Manual of Security Sensitive Microbes and Toxins (Liu,
D., ed.) Pt. 67, CRC-Press Taylor and Francis Group LLC, pp.
775-788.
27.Mistou, M.-Y., Sutcliffe, I. C., and van Sorge,
N. M. (2016) Bacterial glycobiology: rhamnose-containing cell wall
polysaccharides in Gram-positive bacteria, FEMS Microbiol. Rev.,
40, 464-479.
28.Starodumova, I. P., Prisyazhnaya, N. V.,
Tarlachkov, S. V., Dorofeeva, L. V., Vasilenko, O. V., and Evtushenko,
L. I. (2014) Systematics of Rathayibacter: from traditional
approaches till genome analysis, in The First Pushchino
School-Conference “Biochemistry, Physiology and
Biospheric Role of Microorganisms”, IBPM, Russian Academy of
Sciences, Pushchino, pp. 7-10.
Supplementary Figures S1 and S2 (PDF)