Changes in the Receptor-Binding Properties of H3N2 Viruses during Long-Term Circulation in Humans
A. S. Gambaryan1,a,b*, A. Balish2, A. I. Klimov2#, A. B. Tuzikov3, A. A. Chinarev3, G. V. Pazynina3, and N. V. Bovin3
1Chumakov Federal Scientific Center for Research and Development of Immune and Biological Products, Russian Academy of Sciences, 108819 Moscow, Russia2Influenza Branch, Centers for Disease Control and Prevention, GA 30333, Atlanta, USA
3Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, 117997 Moscow, Russia
* To whom correspondence should be addressed.
# Deceased.
Received April 3, 2019; Revised June 5, 2019; Accepted June 15, 2019
It was previously shown that hemagglutinin residues Thr155, Glu158, and Ser228 are crucial for the recognition of Neu5Gc. In this study, we demonstrated that the ability to bind the Neu5Gc-terminated receptor is related to the amino acid 145: viruses of years 1972-1999 with Lys145 bind to the receptor, whereas viruses with Asn145 do not. Sporadic appearance and disappearance of the ability to bind Neu5Gc oligosaccharides and the absence of Neu5Gc in the composition of human glycoconjugates indicate the non-adaptive nature of this ability. It was previously shown that unlike H1N1 viruses, H3N2 viruses of years 1968-1989 did not distinguish between Neu5Acα2-6Galβ1-4Glc (6′SL) and Neu5Acα2-6Galβ1-4GlcNAc (6′SLN). H3N2 viruses isolated after 1993 have acquired the ability to distinguish between 6′SL and 6′SLN, similarly to H1N1 viruses. We found that the affinity for 6′SLN has gradually increased from 1992 to 2003. After 2003, the viruses lost the ability to bind a number of sialosides, including 6′SL, that were good receptors for earlier H3N2 viruses, and retained high affinity for 6′SLN only, which correlated with the acquisition of new glycosylation sites at positions 122, 133, and 144, as well as Glu190Asp and Gly225Asp substitutions, in hemagglutinin. These substitutions are also responsible for the receptor-binding phenotype of human H1N1 viruses. We conclude that the convergent evolution of the receptor specificity of the H1N1 and H3N2 viruses indicates that 6′SLN is the optimal natural human receptor for influenza viruses.
KEY WORDS: influenza virus, hemagglutinin, sialooligosaccharides, receptor specificity, H3N2DOI: 10.1134/S0006297919100067
Abbreviations: CE, embryonated chicken egg; Glyc-PAA, glycosylated polyacrylamide; Glyc-PAA-biotin, biotinylated glycosylated polyacrylamide; HA, hemagglutinin; NA, neuraminidase; RBS, receptor-binding site; Sia, sialic acid (either Neu5Ac or Neu5Gc).
Influenza viruses are negative-strand enveloped RNA viruses with
segmented genomes. They contain eight different RNAs that encode 10 to
11 proteins. The viral particle includes two major envelope
glycoproteins: hemagglutinin (HA) and neuraminidase (NA). The natural
reservoir of influenza viruses are wild aquatic birds, in which viruses
with 16 currently known HA antigenic subtypes and 9 NA subtypes can be
found. The viruses can sometimes transmit from the birds to other wild
and domestic animals. On rare occasions, they adapt to new species and
form stable host-specific lineages. It is believed that all known
lineages of influenza viruses in other species, including humans, have
originated from viruses of wild aquatic birds [1].
Sialic acids are the minimal essential determinants of the cell surface receptors for influenza viruses [2, 3]. Sialyloligosaccharides of cell surface glycoproteins and glycolipids exhibit significant structural diversity. The ability of sialic acids to serve as receptor determinants of influenza viruses may be influenced by the structure of their carbohydrate chain. One of the main characteristics of the receptor specificity of influenza viruses is the ability to discriminate between Neu5Acα2-3Gal and Neu5Acα2-6Gal disaccharide moieties [4, 5]. Thus, non-egg-adapted human influenza A and B viruses strongly bind to Neu5Acα2-6Gal-terminated receptors, but not to the Neu5Acα2-3Gal-terminated ones [6]. In contrast, avian viruses do not bind Neu5Acα2-6Gal irrespectively of their HA subtype and exhibit higher affinity for the Neu5Acα2-3Gal-terminated receptors than for free neuraminic acid (Neu5Ac), which is indicative of specific interactions between the HA receptor-binding site (RBS) and 3-linked Gal. Comparison of the HA amino acid sequences revealed that residues at positions 138, 190, 194, 225, 226, and 228 are conserved in the RBSs of influenza viruses of the H1-H12 subtypes isolated from wild ducks. Amino acids 226 and 228 are replaced in H3 HAs of early human H3N2 viruses, while residues 138, 190, 194, and 225 are replaced in H1 HAs of human H1N1 viruses [7, 8].
Human H1N1 viruses bind to the Neu5Acα2-6Galβ1-4GlcNAc trisaccharide (6′SLN) with a higher affinity than to Neu5Acα2-6Galβ1-4Glc (6′SL), whereas early human H3N2 viruses lacked the ability to distinguish between these two structures [5, 8-10]. After 1992, human influenza H3N2 viruses have lacked some properties; namely, they lost the ability to grow in embryonated chicken eggs (CEs) and to agglutinate chicken red blood cells [11-13].
During the years 1993-2003, several substitutions (e.g., Glu190Asp) have appeared in the HAs of human H3N2 viruses at previously conserved positions responsible for the receptor binding [12]. Ile and then Val have become predominant residues at position 226 of H3 HA instead of Leu, which had been typical for HAs of previous H3N2 human isolates [13-15]. New potential glycosylation sites have emerged at positions 122, 133, and 144. Selection of antigenically distinct H3N2 variants can contribute to the switch in the binding affinity of the viruses. Thus, substitutions of residues 156, 158, 189, and 193 altered the binding to the minor human-type glycans YDS and 6-STF and the swine-specific Neu5Gc-Tn receptor [16].
Later, the Gly225Asp substitution appeared. It is interesting to note that Asp190 and Asp225 are conserved residues responsible for the selectivity of human H1N1 viruses for the Neu5Acα2-6Galβ1-4GlcNAc receptor. It was shown that H3N2 isolates of years 1998 and 1999 exhibited higher affinity to 6′SLN than to 6′SL [17].
Due to the RBS rearrangement, viral adaptation to embryonated chicken eggs via new patterns of amino acid substitutions has become possible [18]. Large-scale mutational analysis of the RBSs of the A/WSN/33 (H1N1) and A/Hong Kong/1/1968 (H3N2) viruses revealed many replication-competent mutants not yet observed in nature [19].
Viruses display year-to-year variations in the binding specificity profiles. The earliest human H3N2 viruses preferentially bound short, branched sialylated glycans, while more recent viruses bound long polylactosamine chains terminating in sialic acid, rather than short sialylated glycans [20]. Glycan binding assay demonstrated that early human H3N2 viruses bound to broad range of human receptor analogs, whereas recent (2006-2011) isolates have a more restricted binding profile and preferentially bind to the branched glycans with extended poly-N-acetyl-lactosamine (poly-LacNAc) chains, a feature shared with HA of the 2009 pandemic H1N1 (Cal/04) virus. These human-type long-branched receptors have a potential to increase the binding affinity by simultaneously interacting with two subunits of the HA trimer [21, 22].
In this study, we investigated the receptor-binding properties of human H3N2 influenza viruses isolated from 1968 to 2003.
MATERIALS AND METHODS
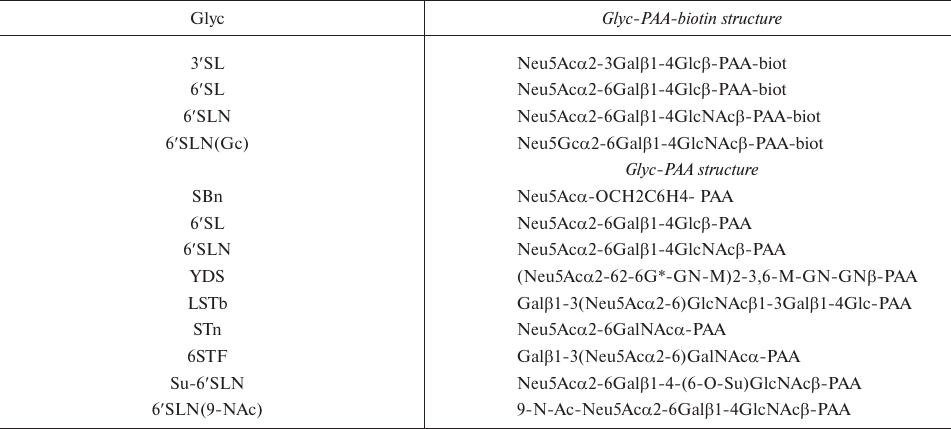
Materials. Horseradish peroxidase–streptavidin conjugate and o-phenylenediamine from Boehringer Mannheim (Germany) and 96-well plates (Costar, USA) were used. α-Benzyl sialoside (SBn) and 6′SLN (9-NAc) were synthesized as described elsewhere [23, 24]. Unlabeled glycosylated polyacrylamides (Glyc-PAAs; 30 kDa) were synthesized as described earlier [25]. Biotinylated Glyc-PAA-biotin polymers (1000 kDa) were synthesized as described in [26]. See Table 1 for the structures and abbreviations of receptor analogs.
Table 1. Structures of
polyacrylamide-attached oligosaccharides
Propagation of viruses. The viruses were obtained from the Centers for Disease Control (Atlanta, GA, USA) virus repository. The viruses were isolated in chicken embryo (E) or in MDCK cells (M) and propagated in MDCK cells. Viruses were grown in MEM medium for 48 h at 37°C and inactivated with 0.1% glutaraldehyde. The cultural liquids were clarified by low-speed centrifugation; the viruses were collected by high-speed centrifugation, resuspended in 0.02 M Tris buffer (pH 7.2) containing 0.1 M NaCl and 50% glycerol, and stored at –20°C.
Direct binding assays. The assay was performed with Glyc-PAA-biotin compounds (1000 kDa) as described in [8]. The plates were coated with purified influenza virus with the titer of 4-8 hemagglutination units (100 µl/well) at 4°C for 2 h, followed by washing with phosphate buffered saline (PBS). Then, 100 µl/well of the blocking solution (0.1% BSA in PBS) was added, and the plates were incubated for 1 h, followed by washing with cold PBS containing 0.05% Tween 20 (washing solution). After addition of 2-fold serial dilutions of Glyc-PAA-biotin (50 μl/well) in the working buffer (PBS with 0.01% Tween 20, 0.1% BSA, and 1 μM neuraminidase inhibitor oseltamivir carboxylate), the plates were incubated for 2 h at 4°C. The starting concentration of Glyc-PAA-biotin was 0.5 μM with respect to sialic acid. The plates were washed with cold washing solution and incubated with streptavidin–peroxidase in the working buffer at 4°C for 1 h. After washing, peroxidase activity in the wells was assayed using o-phenylenediamine solution.
Competitive binding assay. The virus affinity to Glyc-PAA (30 kDa) was determined in the fetuin binding inhibition assay as previously described [27]. The assay is based on the competition between the receptor analog and standard preparation of peroxidase-labeled fetuin for the binding sites on the immobilized virus. The competitive assay was performed for 1 h at 2-4°C in the working buffer. The results were expressed as affinity constants (Kaff) formally equivalent to the dissociation constants of the virus–receptor analog complexes. The constants were calculated based on the concentration of sialic acid residues in the solution; the data from the same set of experiments were averaged.
Statistics. The dissociation constants and standard deviations were calculated with the EXCEL software.
RESULTS
We have studied the receptor-binding properties of human influenza H3N2 viruses isolated in MDCK cells (M) and in chicken embryos (E). For more adequate comparison, egg-adapted viruses were grown and harvested in MDCK cells.
In the first set of experiments, we performed direct binding assay using five labeled receptor analogs: peroxidase-labeled fetuin and four Glyc-PAA-biotin probes (1000 kDa) [Glyc is 3′SL, 6′SL, 6′SLN, and 6′SLN(Gc), respectively] (see Table 1 and Fig. 1 for the Glyc structures and molecular models). The obtained results are shown in Table 2. For the ease of comparison, the data are presented in qualitative form: three asterisks, the strongest binding; two asterisks, one order of magnitude weaker binding; one asterisk, weak binding; minus sign, no binding.
Fig. 1. Structures of tested sialooligosaccharides.
Recognition of the sialic acid linkage type (α2-3 vs. α2-6). In agreement with our earlier studies [10, 17], none of the MDCK-derived viruses bound to 3′SL, while all egg-adapted viruses bound to this receptor analog, despite the fact that they were MDCK-grown at the last passage (Table 2).
Table 2. Virus binding with
sialoglycoconjugates
Note: M and E, human influenza H3N2 viruses isolated in MDCK
cells and chicken Embryos, respectively; Fet, fetuin; nd, not
determined.
Binding to fetuin. Fetuin is a glycoprotein possessing mostly bi- and triantennary N-glycans terminated with Siaα2-3Galβ1-4GlcNAc and Siaα2-6Galβ1-4GlcNAc [28]. All the viruses isolated before 1999 and all egg-adapted viruses bound fetuin with a high affinity. Most of the viruses isolated after 1999 lost this ability, which correlated with the appearance of new glycosylation sites (GS) at positions 122, 133 and 144. The Texas/41/99 and Alaska/37/99 viruses, in which the GS122 was absent, and the A/New York/68/2003 and A/New York/72/2003 viruses that lost the GS144 a second time, partly preserved the affinity to fetuin. The loss of GS126 restored the ability to bind fetuin at a very low level. Hence, the abundance of new glycosylation sites (positions 122, 133, and 144) is crucial for the inability to bind fetuin.
Recognition of the sialic acid type (N-acetylneuraminic vs. N-glycolylneuraminic). No specific pattern in the ability of the tested H3N2 viruses to recognize N-glycolylneuraminic acid was observed. Both early and late viruses with Trp155 lacked the ability to bind 6′SLN(Gc). The A/Port Chalmers/73 virus bound N-acetylneuraminic and N-glycolylneuraminic acids with equal affinity, with is in good agreement with the results of studies [29, 30] demonstrating that the H3N2 viruses with Tyr155 recognize Neu5Gcα2-6Gal. The ability to bind Neu5Gc of the viruses from 1992-1999 correlated with the combination of Lys145 and His155. Viruses with Lys145 bound Neu5Gc, while viruses with Asn145 did not (Table 2). Viruses isolated after 2002 did not bind 6′SLN(Gc) even with Lys145 and His155 in HA, which might be due to further RBS rearrangement, including substitutions of amino acid 190, 225, and 226.
Recognition of 6′SL vs. 6′SLN. Human H3N2 viruses of 1968-2003 demonstrated different affinity to 6′SL compared to 6′SLN. Viruses isolated from 1968 to 1992 recognized these compounds with equal affinity. H3N2 viruses isolated after 1993 bound 6′SLN with a higher affinity than 6′SL. As a rule, viruses isolated after 2002 completely lost the ability to bind 6′SL. The A/New York/68/2003 and A/New York/72/2003 viruses without GS144 are exceptions to this rule; they bound 6′SL at a low level. The first sign of increasing affinity to 6′SLN in comparison with 6′SL was observed for viruses isolated in 1993, in which Glu replaced Asp at position 190 (Table 3). This trend has strengthened after the appearance of new GSs (Table 2). Viruses with GS144 and Gly225/Asp substitution did not bind 6′SL. Nevertheless, their ability to bind to 6′SLN remained elevated.
Virus specificity to Sia(2-6)-terminated receptors with different saccharide cores. The receptor-binding phenotypes of human H3N2 viruses were determined using Neu5Acα2-6Gal receptors differing in their inner core (Table 1 and Fig. 1). Receptor mimetics α-benzyl sialoside (SBn) and 6′SLN (9-NAc) were synthesized; the other sialooligosaccharides were found in natural glycoproteins or glycolipids and isolated from biological materials. Unlabeled sialoglycopolymers with low molecular weight (30 kDa) were used in the competitive assay [25]. The binding affinities for these receptor analogs are presented in Table 3. All the tested viruses bound to 6′SLN and 6-O-Su-6′SLN with equal affinity. None of the viruses bound to 6′SLN(9-NAc) and 6-STF; hence, the data for 6-O-Su-6′SLN, 6′SLN(9-NAc), and 6-STF were omitted.
Table 3. Virus binding affinity
(Kaff, μM) with sialylglycoconjugates
Notes: Affinity constants (Kaff) are formally
equivalent to the dissociation constants of the virus–receptor
analog complexes; higher Kaff values correspond to
lower affinities. The data were averaged from three sets of
experiments; standard deviations did not exceed 60% of the mean values.
M and E, human influenza H3N2 viruses isolated in MDCK cells and
chicken Embryos, respectively; nd, not determined.
The equal affinity to 6′SLN and its sulfated analog 6-O-Su-6′SLN is not surprising, since the sulfo group attached to O-6 of N-acetylglucosamine is directed from the RBS toward water. The absence of binding to 6′SLN(9-NAc) is not surprising either, since the 9-NAc-group cannot interpose between C-9 of Neu5Ac and the surface of receptor-binding pocket in the region of amino acids 183, 190, and 228 [3]. The absence of binding to 6-STF could be explained by disadvantageous overlapping of the Galβ moiety with the 222-226 fragment of the polypeptide chain in the RBS.
All the tested viruses demonstrated high affinity to 6′SLN. The affinity to the 6′SLN-containing biforked oligosaccharide YDS was lower than to 6′SLN. The difference increased with the year of isolation and could be explained by steric overlapping of the YDS second antenna with carbohydrate moieties of HA in the RBS region. The results of the competitive and direct binding assays were the same.
Viruses isolated before 1999 demonstrated nearly equal affinity to 6′SL and 6′SLN, whereas viruses isolated after 1999 had low or no affinity to 6′SL. The affinity to STn and LSTb was similar between all the tested viruses: it was very low for viruses from 1968-1992, up to Umea/02/92 virus. The Qingdao/53/92, Stockholm/1/93, Wyoming/1/93, and Spain/351/95 viruses displayed a high affinity to these two compounds, which was only slightly lower than the affinity to 6′SLN. All these viruses have the Glu190Asp substitution. The Wuhan/359/95 virus (with Leu226Ile substitution) exhibited a strongly decreased affinity to these oligosaccharides. Later viruses with Ile/Val226 and GS133 and GS144, virtually did not bind to STn and LSTb.
It was shown earlier that α-benzyl sialoside (SBn) (Table 1) is a strong inhibitor of binding of human H3N2 viruses [31, 32]. Indeed, for all but three viruses (three last viruses in Table 3), the affinity to SBn was equal to the affinity to 6′SLN. The Wyoming/3/2003, Texas/33/2003, and NewYork/68/2003 viruses demonstrated a dramatically decreased affinity to SBn and 6′SL and had the Gly225Asp substitution in HA. It is likely that bulky hydrophilic Asp residue completely abolished the hydrophobic interaction of the benzyl group with Val226.
DISCUSSION
Herein, we evaluated the evolution of the receptor specificity of 1968-2003 human H3N2 viruses in a context of amino acid substitutions in the RBS and of sialoside core structure. Two replacements, Gln226Leu and Gly228Ser, distinguished HA of early human viruses from the consensus sequence in avian viruses. These substitutions changed the virus preference for the receptor determinant from Neu5Acα2-3Gal to Neu5Acα2-6Gal. However, this preference was not absolute – the affinity to the non-anchored 3′SL was nearly 5 times lower than to 6′SL [31, 32]. The complex of Neu5Acα2-3Gal-terminated receptor with the Aichi/2/68 HA was successfully crystallized, and its three-dimensional structure was determined [33]. Early human H3N2 viruses had been able to bound to Neu5Acα2-3Gal-terminated gangliosides; but this ability has decreased and was lost afterwards [7].
According to Anders and Suzuki, HA amino acids 155, 158, and 228 may be critical for the recognition of Neu5Ac and Neu5Gc [29, 30]. In our study, the ability of viruses isolated in 1992-1999 to bind to Neu5Gc correlated with the pair of amino acids 145 and 155: viruses with Lys145 and His155 bound to the Neu5Gc receptor, while viruses with Asn145 and His155 or Lys145 and Thr155 did not (Table 2). The proposed position of Lys145 relative to the N-glycolyl group of N-glycolylneuraminic acid and Trp153 is shown in Fig. 2. Apparently, the amino-group of Lys145 is able to form a hydrogen bond with the N-glycolyl group.
Fig. 2. Putative position of Lys145 in the RBS of H3 HA. Trp153, Lys145 and N-glycolyl group of sialic acid (SA) are shown. Substitutions of Ser145 with Lys145 and N-acetyl group of SA with N-glycolyl group were performed with the DS ViewerPro 5.0 software (Accelrys Inc.) using the three-dimensional model of X-31 HA complexed with the LSTc pentasaccharide [33].
Sporadic appearance and disappearance of the affinity for Neu5Gc in viruses from 1968-2003 indicates the non-adaptive character of this feature. The Neu5Gc moiety is absent on human cells; hence, the ability to bind this form of sialic acid cannot provide a virus with the selective advantage. Sporadic appearance of this ability seems to be an accidental result of the amino acid replacements in the hypervariable regions of antigenic sites.
Early human H3N2 viruses were not adapted to a certain sialyloligosaccharide in the human respiratory epithelium. Initial Gln226Leu and Gly228Ser substitutions in HA ensured virus interactions with the methylene group of galactose moiety adjacent to 2-6Sia. Receptor mimetics of the general Neu5Acα-OCH2-R structure containing hydrophobic aglycon, such as benzyl sialoside and even α-methyl sialoside, exhibited high affinity to early H3N2 viruses [31]. When the asialic part of the carbohydrate receptor analog did not sterically hinder the binding, different compounds with the 2-6 linked sialic acid demonstrate almost identical high affinity toward the viruses [9, 31]. With the appearance of human subpopulation with the antibodies against H3N2, new glycosylation sites in HA at positions 126 and 246 have appeared in H3N2 viruses, reducing the neutralizing effect of the antibodies. At the same time, some natural receptors have become inaccessible for the viruses. Thus, the ability to bind GM3 and 3-SPG gangliosides decreased with the acquisition of GS126 and disappeared with the acquisition of GS246 [7].
When Glu190 (residue conserved in the avian viruses) was replaced with Asp, at least two new natural sialooligosaccharides (STn and LSTb) became available for the viruses. Viruses with Asp190 and Leu226 bound STn and LSTb with a high affinity, but this ability was lost with the Leu226Ile/Val substitution. Since the residues 190 and 225 are in the close contact the terminal part of the receptor (sialic acid and adjacent galactose fragment), they cannot directly affect distant monosaccharides. It is probable that substitutions at these positions slightly turn the sialic acid, resulting in a more or less successful fitting of STn and LSTb in the RBS.
When STn and LSTb became available for the viruses, 6′SLN became the receptor with the highest affinity, and further evolution promoted this trend. The affinity to STn, LSTb, and 6′SL gradually disappeared, while the affinity to 6′SLN remained high. After Gly225Asp substitution, the original mechanism of receptor recognition (via hydrophobic interaction) was lost, and the viral RBS underwent a deep rearrangement. After 2003, the viruses contain Asp190, Asp225, Val226, and Ser228 in the RBS instead of Leu226 and Ser228, which distinguishes them from the avian viruses.
In conclusion, it appears that over the course of evolution, the receptor phenotypes of human H3N2 and H1N1 viruses have converged, and the amino acid composition of their RBSs has become closer than in the beginning of H3N2 circulation. Both H1N1 and H3N2 viruses of 2003-2009 have numerous glycosylation sites in the upper part of HA, along with Asp at positions 190 and 225, and strongly discriminate between the receptors with 6′SL and 6′SLN. The role of Asp190 and Asp225 in the selective affinity of H1N1 viruses to 6′SLN was shown earlier [10]. Crystallography studies of the H1 HA molecule revealed hydrogen bonds between Asp190 and the amine nitrogen of the GlcNAc-3 moiety of the 6′SLN molecule [34]. Hence, it is possible that a similar mechanism is responsible for the discrimination between 6′SL and 6′SLN in the binding of H3N2 viruses. Such convergent evolution of the receptor specificity of H1N1 and H3N2 subtypes suggests that 6′SLN is the optimal natural human receptor for the influenza viruses.
Funding. This study was supported by the Russian Foundation for Basic Research (projects 02-04-48109, 01-04-49300, 14-04-00547, and 17-04-00148) and the International Science and Technology Center (grant no. 5-2464).
Conflict of interest. The authors declare no conflict of interest in financial or any other sphere.
Acknowledgements. We regret to inform that our colleague Alexander Klimov has died.
Ethical approval. This article does not contain any studies with animals or human participants performed by any of the authors.
REFERENCES
1.Webster, R. G., Bean, W. J., Gorman, O. T.,
Chambers, T. M., and Kawaoka, Y. (1992) Evolution and ecology of
influenza A viruses, Microbiol. Rev., 56, 152-179.
2.Paulson, J. C. (1985) Interactions of animal
viruses with cell surface receptors, in The Receptors (Conn, M.,
ed.) Academic Press, Orlando, FL, Vol. 2, pp. 131-219.
3.Wiley, D. C., and Skehel, J. J. (1987) The
structure and function of the hemagglutinin membrane glycoprotein of
influenza virus, Ann. Rev. Biochem., 56, 365-394; doi:
10.1146/annurev.bi.56.070187.002053.
4.Rogers, G. N., and D’Souza, B. L. (1989)
Receptor-binding properties of human and animal H1 influenza virus
isolates, Virology, 173, 317-322.
5.Connor, R. J., Kawaoka, Y., Webster, R. G., and
Paulson, J. C. (1994) Receptor specificity in human, avian, and equine
H2 and H3 influenza virus isolates, Virology, 205, 17-23;
doi: 10.1006/viro.1994.1615.
6.Gambaryan, A. S., Tuzikov, A. B., Piskarev, V. E.,
Yamnikova, S. S., Lvov, D. K., Robertson, J. S., Bovin, N. V., and
Matrosovich, M. N. (1997) Specification of receptor-binding phenotypes
of influenza virus isolates from different hosts using synthetic
sialylglycopolymers: non-egg-adapted human H1 and H3 influenza A and
influenza B viruses share a common high binding affinity for
6′-sialyl-N-(acetyllactosamine), Virology, 232,
345-350; doi: 10.1006/viro.1997.8572.
7.Matrosovich, M. N., Gambaryan, A. S., Teneberg, S.,
Piskarev, V. E., Yamnikova, S. S., Lvov, D. K., Robertson, J. S., and
Karlsson, K. A. (1997) Avian influenza A viruses differ from human
viruses by recognition of sialyloligosaccharides and gangliosides and
by a higher conservation of the HA receptor-binding site,
Virology, 233, 224-234; doi: 10.1006/viro.1997.8580.
8.Matrosovich, M., Tuzikov, A., Bovin, N., Gambaryan,
A., Klimov, A., Castrucci, M. R., Donatelli, I., and Kawaoka, Y. (2000)
Early alterations of the receptor-binding properties of H1, H2, and H3
avian influenza virus hemagglutinins after their introduction into
mammals, J. Virol., 74, 8502-8512; doi:
10.1128/JVI.74.18.8502-8512.
9.Gambaryan, A. S., Piskarev, V. E., Yamskov, I. A.,
Sakharov, A. M., Tuzikov, A. B., Bovin, N., Nifant’ev, N. E., and
Matrosovich, M. N. (1995) Human influenza virus recognition of
sialyloligosaccharides, FEBS Lett., 366, 57-60.
10.Gambaryan, A. S., Robertson, J. S., and
Matrosovich, M. N. (1999) Effects of egg-adaptation on the
receptor-binding properties of human influenza A and B viruses,
Virology, 258, 232-239; doi: 10.1006/viro.1999.9732.
11.Grassauer, A., Egorov, A. Y., Ferko, B.,
Romanova, J., Katinger, H., and Muster, T. (1998) A host
restriction-based selection system for influenza haemagglutinin
transfectant viruses, J. Gen. Virol., 79, 1405-1409; doi:
10.1099/0022-1317-79-6-1405.
12.Nobusawa, E., Ishihara, H., Morishita, T., Sato,
K., and Nakajima, K. (2000) Change in receptor-binding specificity of
recent human influenza A viruses (H3N2): a single amino acid change in
hemagglutinin altered its recognition of sialyloligosaccharides,
Virology, 278, 587-596; doi: 10.1006/viro.2000.0679.
13.Medeiros, R., Escriou, N., Naffakh, N.,
Manuguerra, J. C., and van der Werf, S. (2001) Hemagglutinin residues
of recent human A (H3N2) influenza viruses that contribute to the
inability to agglutinate chicken erythrocytes, Virology,
289, 74-85; doi: 10.1006/viro.2001.1121.
14.Fitch, W. M., Bush, R. M., Bender, C. A., and
Cox, N. J. (1997) Long term trends in the evolution of H3HA1 human
influenza type A, Proc. Natl. Acad. Sci. USA, 94,
7712-7718; doi: 10.1073/pnas.94.15.7712.
15.Bush, R. M., Bender, C. A., Subbarao, K., Cox, N.
J., and Fitch, W. M. (1999) Predicting the evolution of human influenza
A, Science, 286, 1921-1925.
16.Wang, X., Ilyushina, N. A., Lugovtsev, V. Y.,
Bovin, N. V., Couzens, L., Gao, J., Donnelly, R. P., Eichelberger, M.
C., and Wan, H. (2017) Amino acids in hemagglutinin antigenic site B
determine antigenic and receptor binding differences between A(H3N2)v
and ancestral seasonal H3N2 influenza viruses, J. Virol.,
91, e01512-16; doi: 10.1128/JVI.01512-16.
17.Mochalova, L., Gambaryan, A., Romanova, J.,
Tuzikov, A., Chinarev, A., Katinger, D., Katinger, H., Egorov, A., and
Bovin, N. (2003) Receptor-binding properties of modern human influenza
viruses primarily isolated in Vero and MDCK cells and chicken
embryonated eggs, Virology, 313, 473-480.
18.Stevens, J., Chen, L. M., Carney, P. J., Garten,
R., Foust, A., Le, J., Pokorny, B. A., Manojkumar, R., Silverman, J.,
Devis, R., Rhea, K., Xu, X., Bucher, D. J., Paulson, J. C., Cox, N. J.,
Klimov, A., and Donis, R. O. (2010) Receptor specificity of influenza A
H3N2 viruses isolated in mammalian cells and embryonated chicken eggs,
J. Virol., 84, 8287-8299; doi: 10.1128/JVI.00058-10.
19.Wu, N. C., Xie, J., Zheng, T., Nycholat, C. M.,
Grande, G., Paulson, J. C., Lerner, R. A., and Wilson, I. A. (2017)
Diversity of functionally permissive sequences in the receptor-binding
site of influenza hemagglutinin, Cell Host Microbe, 21,
742-753; doi: 10.1016/j.chom.2017.05.011.
20.Gulati, S., Smith, D. F., Cummings, R. D., Couch,
R. B., Griesemer, S. B., St George, K., Webster, R. G., and Air, G. M.
(2013) Human H3N2 influenza viruses isolated from 1968 to 2012 show
varying preference for receptor substructures with no apparent
consequences for disease or spread, PLoS One, 8, 66325;
doi: 10.1371/journal.pone.0066325.
21.Yang, H., Carney, P. J., Chang, J. C., Guo, Z.,
Villanueva, J. M., and Stevens, J. (2015) Structure and receptor
binding preferences of recombinant human A(H3N2) virus hemagglutinins,
Virology, 477, 18-31; doi:
10.1016/j.virol.2014.12.024.
22.Peng, W., de Vries, R. P., Grant, O. C.,
Thompson, A. J., McBride, R., Tsogtbaatar, B., Lee, P. S., Razi, N.,
Wilson, I. A., Woods, R. J., and Paulson, J. C. (2017) Recent H3N2
viruses have evolved specificity for extended, branched human-type
receptors, conferring potential for increased avidity, Cell Host
Microbe, 21, 23-34; doi:
10.1016/j.chom.2016.11.004.
23.Bovin, N. V., Korchagina, E. Yu., Zemlyanukhina,
T. V., Byramova, N. E., Galanina, O. E., Zemlyakov, A. E., Ivanov, A.
E., Zubov, V. P., and Mochalova, L. V. (1993) Synthesis of polymeric
neoglycoconjugates based on N-substituted polyacrylamides,
Glycoconj. J., 10, 142-151.
24.Shilova, N. V., Galanina, O. E., Pochechueva, T.
V., Chinarev, A. A., Kadykov, V. A., Tuzikov, A. B., and Bovin, N. V.
(2005) High molecular weight neoglycoconjugates for solid phase assays,
Glycoconj. J., 22, 43-51; doi:
10.1007/s10719-005-0280-y.
25.Byramova, N. E., Tuzikov, A. B., and Bovin, N. V.
(1992) A simple procedure for the synthesis of the methyl and benzyl
glycosides of Neu5Ac and 4-epi-Neu5Ac, Carbohydr. Res.,
237, 161-175; doi: 10.1016/S0008-6215(92)84240-S.
26.Matrosovich, M. N., Mochalova, L. V., Marinina,
V. P., Byramova, N. E., and Bovin, N. V. (1990) Synthetic polymeric
sialoside inhibitors of influenza virus receptor-binding activity,
FEBS Lett., 272, 209-212.
27.Gambaryan, A. S., and Matrosovich, M. N. (1992) A
solid-phase enzyme-linked assay for influenza virus receptor-binding
activity, J. Virol. Methods, 39, 111-123.
28.Hayase, T., Rice, K. G., Dziegielewska, K. M.,
Kuhlenschmidt, M., Reilly, T., and Lee, Y. C. (1992) Comparison of
N-glycosides of fetuins from different species and human alpha
2-HS-glycoprotein, Biochemistry, 31, 4915-4921.
29.Anders, E. M., Scalzo, A. A., Rogers, G. N., and
White, D. O. (1986) Relationship between mitogenic activity of
influenza viruses and the receptor-binding specificity of their
hemagglutinin molecules, J. Virol., 60, 476-482.
30.Suzuki, Y. (2001) Host mediated variation and
receptor binding specificity of influenza viruses, Adv. Exp. Med.
Biol., 491, 445-451.
31.Matrosovich, M. N., Gambaryan, A. S., Tuzikov, A.
B., Byramova, N. E., Mochalova, L. V., Golbraikh, A. A., Shenderovich,
M. D., Finne, J., and Bovin, N. V. (1993) Probing of the
receptor-binding sites of the H1 and H3 influenza A and influenza B
virus hemagglutinins by synthetic and natural sialosides,
Virology, 196, 111-121; doi: 10.1006/viro.1993.1459.
32.Pritchett, T. J., Brossmer, R., Rose, U., and
Paulson, J. C. (1987) Recognition of monovalent sialosides by influenza
virus H3 hemagglutinin, Virology, 160, 502-506.
33.Eisen, M. B., Sabesan, S., Skehel, J. J., and
Wiley, D. C. (1997) Binding of the influenza A virus to cell-surface
receptors: structures of five hemagglutinin-sialyloligosaccharide
complexes determined by X-ray crystallography, Virology,
232, 19-31; doi: 10.1006/viro.1997.8526.
34.Gamblin, S. J., Haire, L. F., Russell, R. J.,
Stevens, D. J., Xiao, B., Ha, Y., Vasisht, N., Steinhauer, D. A.,
Daniels, R. S., Elliot, A., Wiley, D. C., and Skehel, J. J. (2004) The
structure and receptor-binding properties of the 1918 influenza
hemagglutinin, Science, 303, 1838-1842; doi:
10.1126/science.1093155.