REVIEW: Epigenetic Mechanisms Involved in the Effects of Maternal Hyperhomocysteinemia on the Functional State of Placenta and Nervous System Plasticity in the Offspring
Alexander V. Arutjunyan1,2,a*, Yulia P. Milyutina1,3, Anastasia D. Shcherbitskaia1,4, Gleb O. Kerkeshko1,2, and Irina V. Zalozniaia1
1Ott Research Institute of Obstetrics, Gynecology and Reproductive Medicine, 199034 St. Petersburg, Russia2St. Petersburg Institute of Bioregulation and Gerontology, 197110 St. Petersburg, Russia
3St. Petersburg State Pediatric Medical University, 194100 St. Petersburg, Russia
4Sechenov Institute of Evolutionary Physiology and Biochemistry, Russian Academy of Sciences, 194223 Saint Petersburg, Russia
* To whom correspondence should be addressed.
Received November 23, 2022; Revised February 2, 2023; Accepted February 2, 2023
According to modern view, susceptibility to diseases, specifically to cognitive and neuropsychiatric disorders, can form during embryonic development. Adverse factors affecting mother during the pregnancy increase the risk of developing pathologies. Despite the association between elevated maternal blood homocysteine (Hcy) and fetal brain impairments, as well as cognitive deficits in the offspring, the role of brain plasticity in the development of these pathologies remains poorly studied. Here, we review the data on the negative impact of hyperhomocysteinemia (HHcy) on the neural plasticity, in particular, its possible influence on the offspring brain plasticity through epigenetic mechanisms, such as changes in intracellular methylation potential, activity of DNA methyltransferases, DNA methylation, histone modifications, and microRNA expression in brain cells. Since placenta plays a key role in the transport of nutrients and transmission of signals from mother to fetus, its dysfunction due to aberrant epigenetic regulation can affect the development of fetal CNS. The review also presents the data on the impact of maternal HHcy on the epigenetic regulation in the placenta. The data presented in the review are not only interesting from purely scientific point of view, but can help in understanding the role of HHcy and epigenetic mechanisms in the pathogenesis of diseases, such as pregnancy pathologies resulting in the delayed development of fetal brain, cognitive impairments in the offspring during childhood, and neuropsychiatric and neurodegenerative disorders later in life, as well as in the search for approaches for their prevention using neuroprotectors.
KEY WORDS: maternal (prenatal) hyperhomocysteinemia, epigenetic regulation, fetus, newborn, brain, placenta, neuronal plasticityDOI: 10.1134/S0006297923040016
Abbreviations: AD, Alzheimer’s disease; BDNF, brain-derived neurotrophic factor; DNMT, DNA methyltransferase; Hcy, homocysteine; HDAC, histone deacetylase; HHcy, hyperhomocysteinemia; IUGR, intrauterine growth restriction; LINE-1, long interspersed nuclear element; LTP, long-term potentiation; MeCP2, methyl-CpG-binding protein 2; NDD, neurodegenerative disease; NMDA, N-methyl-D-aspartate; PE, preeclampsia; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine.
INTRODUCTION
Maternal, or prenatal, hyperhomocysteinemia (HHcy) is characterized by increased levels of the non-proteinogenic sulfur-containing amino acid homocysteine (Hcy) during pregnancy. It can result in the altered structure and function of cells, tissues, organs, and organ systems, as well as impairments in fetal homeostasis in embryogenesis followed by persistent negative consequences at the postnatal stage of ontogenesis [1-4]. Numerous experimental studies have shown that maternal HHcy can impair brain development in a fetus or a newborn [5-23], thus affecting neurobehavioral development and cognitive functions of the offspring [5, 6, 10, 12, 15, 18, 20, 24-38]. Multiple clinical data indicate that an increased Hcy level in the mother’s blood can be associated with the risk of congenital malformations of the fetal CNS [39, 40]. Recent clinical studies have established a relationship between the elevated Hcy levels in mother’s blood and decline of cognitive performance and intelligence in children [41-44].
Hcy and its metabolites demonstrate pronounced neurotoxic properties; the ability of Hcy to affect the nervous system in general and nerve cells in particular has been reported in many studies [45, 46]. At the cellular level, the cognitive decline in the offspring due to maternal HHcy can be associated with a decrease in specific neuronal subpopulations in the brain caused by the activation of apoptosis, delayed migration of neurons, impaired synthesis of neurotransmitters and formation of synapses, and development of neuroinflammation associated with the activation of astrocytes and microglia [4]. However, the molecular mechanisms underlying these processes remain poorly investigated. The most studied mechanisms of Hcy neurotoxicity include the development of oxidative stress [47-49], excitotoxicity related to overactivation of N-methyl-D-aspartate (NMDA) and metabotropic glutamate receptors due to the structural similarity of Hcy to glutamate [45, 50, 51], and N-homocysteinylation of proteins that impairs their structure and functions [52, 53]. Despite that the Hcy potential ability to influence epigenetic mechanisms has been well established, the role of these mechanisms in the neurotoxic and other negative effects of HHcy on the developing brain is still obscure.
HHcy-INDUCED EPIGENETIC MODIFICATIONS
Since Hcy is formed via demethylation of the essential amino acid methionine, which is consumed with food proteins, its metabolism is linked with methionine and folate biochemical cycles [54]. In the methionine cycle, methionine adenosyltransferase converts methionine to S-adenosylmethionine (SAM) that serves as a universal donor of methyl groups in the methylation of a wide range of substrates, including DNA, RNA, histones, phospholipids, catecholamines, etc. As a result of methylation reactions catalyzed by methyltransferases with different substrate specificity, SAM is converted into S-adenosylhomocysteine (SAH), which is hydrolyzed to Hcy by S-adenosylhomocysteine hydrolase. SAH suppresses the activity of methyltransferases by the product inhibition mechanism; therefore, the intracellular SAM/SAH ratio is considered as an indicator of cellular methylation potential [55, 56]. The reaction of SAH hydrolysis is reversible and its equilibrium is shifted in the opposite direction [56]. To proceed towards the Hcy formation, levels of Hcy in the cells should be kept low, which in most organs and tissues is achieved by Hcy remethylation to methionine, as well as by its export out of the cell [57]. It was found that the total blood Hcy level correlates with the intracellular SAH content; therefore, it is assumed that an increase in blood Hcy concentration through an increase in SAH can result in a decrease in the activity of methyltransferases in cells and thus, in the inhibition of DNA methylation [56]. The reaction of Hcy remethylation with the formation of methionine links the methionine cycle to the folate cycle, because the active form of folic acid 5-methyl-tetrahydrofolate serves as a source of methyl groups in this reaction. The reaction is catalyzed by methionine synthase, which uses vitamin B12 as a co-enzyme. In some tissues, Hcy is remethylated in a folate-independent pathway that uses as a donor of methyl groups the amino acid betaine, which is consumed with food or is synthesized in the body from choline. The excess of the blood Hcy is sequestered in the liver and kidneys through the catabolic pathway of its transsulfuration to cysteine by cystathionine β-synthase and cystathionine γ-lyase with pyridoxal phosphate (vitamin B6) as a cofactor. Disruptions or imbalance of the methionine and/or folate cycles due to a decrease in the availability levels of methyl donors (folic acid, vitamin B12, betaine, choline, etc.), as well as genetically determined reduction in the activity of Hcy transsulfuration or folate metabolism enzymes, can cause HHcy [58].
Recently, the role of HHcy in various epigenetic modifications, especially in DNA and histone methylation, has been actively investigated [56, 59-62].
HHcy and DNA methylation. During embryonic development, each cell, tissue, and organ acquire specific transcription patterns in a process mediated by epigenetic modifications, in particular, DNA methylation [63]. Changes in the level of DNA methylation are one of the principal epigenetic mechanisms involved in the regulation of gene activity [64]. It is believed that methylation inhibits, whereas demethylation activates gene transcription [65, 66]. In vertebrates, DNA methylation participates in numerous processes associated with the embryonic development, such as regulation of expression of developmental genes, maintenance of genome stability, X-chromosome inactivation, and genome imprinting [67]. DNA is methylated by enzymes of the DNA methyltransferase (DNMT) family. DNMT1 acts as a maintenance methylation agent for a newly synthesized DNA chain in the process of replication, while other members of the DNMT family (DNMT3a and DNMT3b) are expressed exclusively during the embryonic development and are responsible for the establishment of de novo DNA methylation patterns [62].
DNMTs methylate DNA by adding methyl group to the cytosine residue in the CpG dinucleotide. The total level of DNA methylation is estimated by assessing the content of 5-methylcytosine in DNA (global DNA methylation) or the extent of methylation of CpG-rich DNA sequences, specifically, LINE-1 (long interspersed nuclear element 1) retrotransposon. The extent of methylation of individual genes is determined from the level of CpG methylation in their promotors [68].
It has been believed for a long time that DNA methylation is irreversible and that genes methylation patterns established de novo during the embryonic development are maintained throughout entire lifetime. The discovery of DNA demethylation and, therefore, the reversible nature of methylation, was a major breakthrough in understanding of the processes of epigenetic regulation. DNA demethylation is catalyzed by translocation methylcytosine dioxygenases of the TET family, which convert 5-methylcytosine to 5-hydroxymethylcytosine [69, 70]. 5-Hydroxymethylcytosine has immediately become the focus of attention of neuroscientists because it was found to be an epigenetic hallmark characteristic of the CNS as compared to other organs [71]. It was identified mostly in differentiated postmitotic neurons, which is in accordance with a rapid increase in the 5-hydroxymethylcytosine content during synaptogenesis and maturation of neurons in a postnatal brain [72, 73].
The functional consequences of the reduced methylation potential of cells caused by the increase in the SAH content are significant and include demyelination in the CNS [74], decrease in the synthesis of neurotransmitters [75], changes in the membrane phospholipid composition [74], and impaired gene expression and cell differentiation [56, 62]. HHcy leads to the elevation in the content of intracellular SAH (DNMT inhibitor), thus suggesting that HHcy can reduce the extent of DNA methylation in the cells, inducing expression of specific genes. Indeed, in vitro studies in neuronal stem cells demonstrated that Hcy can cause an increase in the intracellular SAH level and decrease in the SAM/SAH ratio (i.e., methylation potential), which is accompanied by a decrease in the activity and expression of DNMTs and DNA hypomethylation [61]. However, in some studies, HHcy and/or folate deficiency did not cause any decrease in the total DNA methylation level or even increased it, followed by the increase in the methylation of certain genes and upregulation of the mRNA and protein expression for some DNMTs [62, 76, 77]. Most likely, the effect of HHcy on the DNA methylation and gene expression depends on a substantially larger set of factors, including the type of cells, intracellular levels of SAM, SAH and other components of the methionine and folate cycles, activity of enzymes involved in these cycles, DNMTs protein levels and activity, etc. This might explain the discrepancies in the data obtained in in vitro and in vivo experiments as well as in different HHcy models, e.g., those involving methionine or Hcy administration, methyl donor-deficient diets, animals with mutations in the genes encoding Hcy metabolism enzymes, etc. [62, 78, 79].
A significant number of studies examined the effects of HHcy on the methylation processes in the brain of adult animals as a model of neurodegenerative changes in adults and in old age. These data show that chronic HHcy affects the content of DNMTs, total DNA methylation level, methylation of CpG-rich regulatory regions and promotors of specific genes, which according to the authors’ opinion can lead to the development of neurodegenerative disorders (NDDs) [80-83]. It was suggested that HHcy disturbs the normal function of the brain–blood barrier (BBB) by affecting methylation of DNA and histones, as well as expression of microRNAs [82].
DNA methylation is critical for the development of fetal brain and survival of neurons in the postnatal period. Mice carrying 95% hypomethylated cells in the brain due to the conditional DNMT1 gene knockout died immediately after birth, while animals with ~30% hypomethylated brain cells survived, however, the hypomethylated cells died rapidly during first three weeks of postnatal life [84]. Decreased DNMT1 expression in the precursor cells in the developing mouse brain negatively affected maturation and survival of neurons and promoted differentiation of astroglia [85].
During pregnancy, when DNA methylation patterns in the fetal epigenome are established, the influence of factors that change the bioavailability and/or transfer of methyl groups can lead to irreversible changes in the fetal epigenome. It is assumed that disorders in the development of the fetal nervous system, including congenital neural tube defects (NTDs), the risk of which increases upon reduction in the folate concentration and increase in the Hcy content in mother’s blood, may be associated with the epigenetic mechanisms regulating proliferation and differentiation of neurons [63, 86]. The brains of fetuses with NTDs were characterized by a decreased total DNA methylation level, as well as reduction of the LINE-1 mobile genome elements methylation and upregulation of their transcription, which induced chromosome instability presumably associated with the emergence of NTDs. Reduced DNA methylation in the brain of children with NTDs correlated with the decreased levels of folic acid and vitamin B12 in the mother’s blood [86].
The effects of maternal HHcy on DNA methylation in the fetal/neonate brain are poorly investigated in experimental models. The study [18] revealed no differences between total DNA methylation levels in the brains of offspring from female mice that fed with folate-normal, folate-deficient and folate-excess diet during pregnancy, indicating the existence of homeostatic mechanisms maintaining a relatively stable level of DNA methylation in developing brain. When the content of methyl group donors (folates, vitamin B12, and choline) in the food was significantly reduced, pregnant rats developed HHcy accompanied by a decrease in the total DNA methylation level and in the SAM/SAH ratio in the fetal brain [9]. A deficit of folates in the diet of pregnant rats also decreased the total level of DNA methylation, total DNMT activity, and expression of DNMT1, DNMT3a, and DNMT3b mRNAs and proteins in the brain of newborn pups [87].
Recent genome-wide analysis of methylation of gene promotors in the brain of newborn mice from the mothers that received folate-deficient and folate-supplemented diet revealed that the effects of this methyl donor on the methylation level in the brain were not unidirectional and depended on specific DNA regions. In both folate-deficient and folate-supplemented diet groups, some gene promotors were hypomethylated, whereas other promoters were hypermethylated, although in the group with folate deficiency, the number of hypomethylated DNA regions was higher than the number of hypermethylated ones [88]. Folate deficiency in the mother’s blood affected brain-specific methylation of multiple genes involved in the nervous system development, neuronal plasticity, and learning/memory, including those related to the neurotrophin signaling pathways [88].
We have previously shown that maternal HHcy causes an increase in the brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) precursors forms content in rat placenta and fetal brains [11]. BDNF is critically important for neurogenesis and neuronal survival; it is also involved in synaptogenesis, synaptic plasticity, and memory consolidation. It was found that some neurological disorders are associated with the impaired epigenetic regulation of the BDNF gene activity [89, 90]. Thus, increased methylation of the BDNF promotor region represses gene transcription due to recruitment of repression complex including the methyl-CpG-binding protein 2 (MeCP2) and histone deacetylases (HDACs), which promotes chromatin transition to the compact inactive state. Neuronal activity causes a decrease in the BDNF promotor methylation and dissociation of repression complex from the BDNF promotor that results in active chromatin remodeling and subsequent BDNF gene activation [91, 92], which leads to an increase in the synthesis of BDNF protein involved in neuronal plasticity [93]. The effects of HHcy on the epigenetic regulation of BDNF and NGF neurotrophins genes remain poorly studied, although such effects have been proposed before [94, 95]. Adverse environmental conditions including stress, hypoxia, insufficient diet and exposure to toxins can affect BDNF gene expression through changes in DNA methylation and histone modifications [89, 96]. The involvement of these mechanisms in HHcy has been supported by the data on the upregulated methylation and decreased expression of BDNF in the hippocampus after a single administration of methionine to adult male rats [97]. In mice, a maternal methyl donor-deficient diet (folic acid, vitamins B6 and B12) led to a decrease in the BDNF level in the hippocampus of adult offspring [98]. On the other hand, addition of folic acid to the pregnant mice diet prevented an increase in the methylation level and decrease in the BDNF expression in the hippocampus of the offspring induced by a high-fat maternal diet [99].
These data indicate a vital role of DNA methylation in the programming of normal nervous system development in early ontogenesis and demonstrate that maternal HHcy can negatively affect the development of fetal brain by compromising DNA methylation.
HHcy and posttranslational histone modifications. Histone methylation modifies chromatin structure and determines the accessibility of DNA regulatory elements to transcription factors, thus affecting gene expression [100]. The studies of mono- (me), di- (me2), and trimethylation (me3) of lysine residues in the core histones H3 and H4 are of particular importance because impairments in methylation of these histones are believed to be associated with developmental defects and some types of cancer [100]. Methylation of lysine residues in histones can activate or repress transcription, depending on the lysine position and its methylation level. As a rule, methylation of histone H3 at Lys4, Lys36, and Lys79 residues (H3K4, H3K27, and H3K79, respectively) is considered as a marker of active transcription, whereas H3K9, H3K27 or H4K20 are markers of transcriptionally inactive chromatin [101]. The regulation of histone H3 methylation at Lys4 (H3K4) is especially interesting, because its impairment in the CNS is associated with various neurological diseases [102]. Methylation of H3K4 is a marker of upregulated transcriptional activity of genes involved in neurogenesis, whereas inhibition of H3K4 trimethylation (H3K4me3) decreases expression of these genes, indicating an important role of histone methylation in the development and functioning of neurons in the CNS [96]. Since SAM is a substrate of histone methyltransferases, while SAH inhibits their activity, the level of Hcy can influence histone methylation [62]. Recent studies suggest that the methionine cycle acts as the key metabolic sensory system mediating receptor-independent recognition of metabolism-related signals, as well as modulating SAM/SAH-dependent histone methylation under physiological conditions and during the development of various diseases [103, 104].
Histone acetylation/deacetylation is another important type of epigenetic regulation [62]. Histone acetylation negatively correlates with DNA methylation [105]. Histones acetylation at lysine residues is catalyzed by acetylase enzymes, it results in the addition of a negative charge to histones and is characteristic of transcriptionally active, non-compact chromatin [106]. Histones are deacetylated by HDACs, resulting in chromatin condensation and inactivation of gene transcription [107]. Hence, acetylation of nucleosomal histones plays an important role in switching between permissive and repressive forms of chromatin.
Histone modifications, primarily methylation and acetylation, are essential in neurogenesis and neuronal plasticity [96], as they affect neuronal differentiation, axonal growth, and density and shape of dendritic spines via changes in the expression of specific neuronal genes. Histone modifications in complex with other epigenetic mechanisms regulate expression of synaptic proteins and proteins involved in synaptic plasticity, such as BDNF [89, 96].
There is no evidence of direct Hcy involvement in histone acetylation; however, it was suggested that by altering DNA methylation, Hcy can affect the formation of complexes between HDACs and methyl-CpG-binding proteins, specifically MeCP2, and thus, modulate histone acetylation [62]. Hcy can also disrupt histone modification by N-homocysteinylation of lysine residues in histones, thus preventing their normal acetylation/methylation [53]. The data obtained in cell cultures demonstrated that HHcy not only affects DNA methylation, but also alters expression of methyltransferases and acetyltransferases, which modify histones [108]. In mouse heart tissue, HHcy decreased the activity of HDAC1 and increased histone H3 acetylation at Lys9 (H3K9ac) [109]. Chronic HHcy increased the content of H3K9ac and histone H4 acetylated at Lys12 (H4K12ac) in mouse cerebral cortex, which was probably associated with the neuroinflammatory processes induced by Hcy [110]. HHcy-related DNA hypomethylation and histone hyperacetylation, which cause untimely activation of transcription of various genes, can make brain tissue more susceptible to various lesions, in particular, those caused by ischemia–reperfusion [110]. Methionine administration enhanced methylation of the promotor of the RELN gene encoding the reelin protein in the mouse brain, followed by decreased expression of reelin responsible for the regulation of neuronal migration, formation of cortical layer structure in the developing brain, and modulation of synaptic plasticity. Administration of HDAC inhibitors increased the level of histone H3 acetylation and decreased methylation of the RELN gene in the mouse brain [111, 112].
Fetal hypoxia resulting from placental insufficiency may impair the development of fetal nervous system due to the adverse effects on proliferation and differentiation of nerve and endothelial cells via various epigenetic mechanisms, including DNA methylation, histone modification, and microRNA expression [113, 114]. In the model of intrauterine growth restriction (IUGR) caused by placental insufficiency accompanied by hypoxia, rat offspring showed an increase in the Hcy and SAH levels, DNA hypomethylation, a decrease in the DNMT1 expression, and a significant increase in the content of acetylated histone H3 in the liver [115]. Imbalanced maternal diet can also negatively affect the development of offspring via epigenetic mechanisms dependent on the metabolic signals. It was mentioned above that the high-fat maternal diet led to the hypermethylation and decreased activity of genes encoding BDNF and NR2B subunit of the NMDA receptor (GRIN2B) in the offspring brain [99]. The offspring of female rats fed with a low-isoflavone soy protein diet during pregnancy, exhibited increased Hcy levels in the blood serum accompanied by DNA hypomethylation, decreased content of histone H3 trimethylated at Lys9 (H3K9me3), and increased content of H3K9ac in the liver [116].
Therefore, in addition to changes in the extent of DNA methylation, maternal HHcy can affect expression of genes controlling the development of fetal brain through the post-translational histone modification (methylation and acetylation) and direct Hcy interaction with histone molecules (homocysteinylation).
HHcy and microRNA expression. The effects of HHcy on the expression of microRNAs are poorly studied compared to its influence on DNA methylation or histone modification.
HHcy can disturb the function of the BBB not only through the changes in the DNA and histones methylation, but also by affecting microRNAs levels in the brain. It has been shown that HHcy induces an increase in the expression of more than twenty microRNAs in brain endothelial cells with the maximum increase in levels of miR29b, which causes a decrease in the content of DNMT3b and subsequent increase in the activity of MMP-9 metalloproteinase, leading to the impairment in the BBB integrity [82]. Maternal HHcy caused by the methyl donor-deficient diet led to the developmental disorders of rat fetuses and increased levels of let-7a and miR-34a microRNAs in the fetal brain. Expression of these microRNAs depended on the degree of DNA methylation [9]. It is believed that both let-7a and miR-34a are involved in various stages of brain development and may play a role in the emergence of NTDs. Let-7 affects regulatory pathways controlling proliferation and differentiation of nerve cells, i.e., processes closely related to the NTD development. The target genes of miR-34a are involved in the regulation of cell cycle, apoptosis, cell differentiation, and maintenance of neuronal functions. An increase in the miR-34a level in the brains of newborn rats whose mothers received folate-deficient diet, was accompanied by the activation of apoptosis in the cortex and hippocampus [19]. By the time of weaning, the offspring of mothers with the nutritional methyl donor deficiency demonstrated a decrease in the content of miR-34a in the brain, as well as of miR-23, which, in addition to regulating cell proliferation and apoptosis, is a key factor of myelination [10].
These data show a possible effect of maternal HHcy on epigenetic regulation (DNA methylation, histone modification, and microRNA expression) in various tissues of developing fetus, including its brain. Along with altering epigenetic mechanisms in the fetal CNS, maternal HHcy can affect epigenetic regulation of the formation and functioning of placenta, the transient organ that plays a key role in a fetal development.
Epigenetic modifications in the placenta during HHcy. It is believed that many neurobehavioral defects in the offspring are caused by pathophysiological alterations in the mother’s placenta during fetal development, including those mediated by epigenetic mechanisms [117, 118]. Epigenetic processes play a significant role in the placenta formation by participating in the trophoblast cells proliferation, migration and invasion, as well as in placental angiogenesis [119, 120]. Placenta produces specific factors and controls nutrient and oxygen delivery to the fetus, which is one of the regulatory mechanisms of the fetal brain development [118]. Such close relationship between the placenta and fetal brain has led to the coinage of the term, the placenta–brain axis [118]. The studies on the interaction between the placenta and fetal brain in the prenatal period might open new opportunities for early diagnosis and treatment of neurobehavioral disorders of the postnatal period [117, 118]. However, the specific mechanisms by which, in response to the changing fetal demands in nutrients and oxygen, the expression of specific genes in the placenta can epigenetically change, and, in turn, possible impairments of epigenetic regulation in the placenta under the influence of adverse factors can cause negative consequences for the morphological and functional development of fetal brain, are still poorly understood [117].
The changes in the pool of methyl group donors during pregnancy can lead to the impairment of epigenetic modification of macromolecules in the placenta. Thus, an excess of Hcy and a deficit of folates and other methyl group donors alter gene expression in the placenta and fetus due to the changed profile of DNA methylation [63, 86, 121].
Folate deficiency impairs decidualization of stromal cells of the uterine endometrium that plays an important role in the embryo survival at the early stages of implantation [122]. Impaired decidualization of the maternal endometrium due to the folate deficiency is followed by multiple changes in the methylation profiles of genes, including those involved in the process of decidual transformation [122].
The effect of blood folate and Hcy levels on total DNA methylation in placenta was studied in pregnant rats [123]. Combined consumption of Hcy and folic acid did not affect the main indices of one-carbon metabolism (the contents of SAM and SAH and the SAM/SAH ratio) in the mothers’ liver and placenta, despite an increase in the Hcy concentration in the blood. However, the level of total DNA methylation in the placenta of animals of this group was significantly higher compared to the animals receiving folic acid only [123]. At the same time, folic acid-deficient diet and a high Hcy content caused a decrease in the methylation potential, which was manifested as an increase in the SAH level in the mother’s liver, a decrease in the SAM content in the placenta, as well as a decrease in the SAM/SAH ratio in both these organs with a concomitant decrease in placental DNA methylation. The level of total placental DNA methylation positively correlated with the folate content in the blood and placenta tissue and inversely correlated with the Hcy concentration in the blood [123]. Women with a genetically reduced activity of folate biosynthesis enzyme methylene tetrahydrofolate reductase demonstrated similar negative association of placental DNA methylation level with the Hcy blood content and positive correlation of this parameter with the blood folate concentration [124]. The extent of placental DNA methylation can be determined not only by the folate and Hcy levels, but also by the vitamin B12 concentration in maternal blood, since it was found that the excess of folate with simultaneously reduced vitamin B12 content also caused a decrease in total DNA methylation in the rat placenta [125]. Therefore, the effect of HHcy on DNA methylation may differ depending on the maternal folate and vitamin B12 status. An increase in the Hcy level in the presence of sufficient amounts of these micronutrients in the blood can intensify methylation presumably due to the increase in the level of blood methionine, which serves as a source of methyl groups. On the other hand, a higher content of Hcy in the blood with simultaneous folate and/or vitamin B12 deficiency increases the level of SAH (inhibitor of methyltransferases) and decreases the level of SAM (methyl group donor), which might cause inhibition of DNA methylation in the placenta.
In a model of IUGR induced by a folate-deficient diet, female mice exhibited a decreased SAH content in the blood, decreased total DNA methylation and LINE-1 retrotransposon methylation in the placenta and fetal brain, and increased content of the ORF1p protein encoded by LINE-1 in fetal tissues [126]. The authors suggested that folate deficiency can stimulate retrotransposition within the genome via demethylation and activation of LINE-1, leading to the genome instability and, eventually, to the IUGR development [126].
Since the studies investigating direct relationship between the levels of Hcy and/or folate in the mother’s blood and epigenetic changes in the placenta are extremely scarce, we found it necessary to provide the data on the epigenetic changes in the placenta in preeclampsia (PE), a pathology of pregnancy usually accompanied by an increased level of Hcy in the maternal blood [127]. It was found that PE was associated with a decrease in the total DNA methylation in the placenta [128]. Women with a more severe form of early-onset PE demonstrated a decrease in the SAM content and in the placental growth factor gene PLGF methylation level in the placental tissue, as well as a negative correlation between these two indices suggesting a causal relationship between them [60]. It should be noted that PlGF synthesized by the placenta reaches the fetal brain and acts as an important regulator of brain angiogenesis [129]. The studies on the role of epigenetic mechanisms in the PE often pay special attention to the epigenetic regulation of the extravillous trophoblast invasion, as impairments in this process are considered as one of the main causes of PE development. It was found that the intake of folic acid, the level of which is usually reduced in PE, increased the invasive potential of placental trophoblast cells [130], as well as upregulated the activity of genes promoting cell invasion and decreased the activity of genes suppressing this process. In many cases, the shifts in the gene activity were accompanied by respective changes in the level of CpG methylation in the corresponding promoter regions [130]. In women with PE, the levels of H3K4me3 and H3K9ac epigenetic marks in the extravillous trophoblast cells were decreased, which may be one of the causes or indications of the impaired invasion process [131]. In the endothelial cells isolated from placentas of women with PE, the content of methylated histones H3K9me2 and H3K9me3 was increased, presumably, due to the development of hypoxia and oxidative stress [132].
Among other epigenetic mechanisms, maternal HHcy can also affect placental functions by altering expression of specific microRNAs. A significant number of microRNAs found in the placenta are expressed from a cluster of genes located on chromosome 19 (C19MC). In the human placenta, expression of the C19MC cluster is detected as early as at the fifth week of pregnancy [133]. Overexpression of the C19MC cluster leads to a decrease in the trophoblast cell migration, which suggests involvement of this cluster in the inhibition of cell migration, reduction of spiral artery remodeling, and placental ischemia in PE [134]. Another microRNA cluster found in the placenta is located on chromosome 14 (C14MC) and encodes several types of microRNAs involved in the regulation of key physiological processes, such as immune suppression and anti-inflammatory and anti-hypoxic responses [135]. Unlike C19MC, expression of microRNAs of the C14MC cluster decreases with the progression of pregnancy, indicating that the controlled increase and/or decrease in the expression of numerous microRNAs during pregnancy contributes to the proper development and functioning of the placenta [136, 137].
Most studies on microRNAs in the placenta have been focused on their role in PE pathogenesis. In many studies, the activity of miR-210, a key factor of hypoxia response, was constitutively elevated in the placenta and plasma in PE; hence, this microRNA together with miR-155, which regulates placental angiogenesis, are considered as predictors of PE development [138, 139]. This is in accordance with the idea that hypoxia-inducing anomalies of placental angiogenesis are among the factors causing PE development. It is assumed that alterations in the array of microRNAs and changes in their content can suppress proliferation and invasion of trophoblast cells and impair angiogenesis in PE [140-142]. In addition to the increased levels of miR-210 and miR-155, more than ten other microRNAs associated with oxidative stress, including miR-29b, were observed in the placenta in PE [143]. An increase in the miR-29b content stimulated apoptosis in cultured trophoblast cells and decreases their capability for invasion and angiogenesis [141]. On the other hand, a decrease in the miR-29b level in the trophoblast cells resulted in a significant upregulation of the DNMT1, DNMT3A, DNMT3B, and SIRT1 (histone deacetylase sirtuin 1) genes expression, accompanied by the increase in the total DNA methylation and decrease in the total protein acetylation [144].
Dysregulation of epigenetic mechanisms (DNA methylation, histone modification, and microRNA expression), which play a significant role in the morphological and functional placental maturation, in maternal HHcy may be one of the reasons of incomplete placental vascular network formation, as well as impaired transport and secretory placental functions followed by a delay in the growth and development of the fetal CNS.
EPIGENETIC MODIFICATIONS AND BRAIN PLASTICITY
It has been commonly believed that the long-term memory is stored as stable changes in the synaptic connections formed after learning. This hypothesis on synaptic plasticity as a basis of memory has been supported by vast experimental data collected over more than 50 years of studies that had started in 1970s. Now, it has become evident that long-term memory is mainly stored as epigenetic and genomic modifications in the nerve cells, while synaptic plasticity provides rapid, but relatively short-term (maximum of several hours) storage of the acquired information. It is assumed that new memory is stored as synaptic changes until its significance for the organism is evaluated. If its significance is higher than a certain threshold level, the memory is transmitted to the neuronal nuclei for the long-term storage [145].
Multiple studies support that epigenetic regulation of gene expression in the neuronal plasticity plays a substantial role in the formation and consolidation of learning and memory, and DNA methylation and histone modification are the most important mechanisms of such neuroepigenetic regulation [96, 146]. Epigenetic mechanisms, such as DNA methylation and histone methylation/acetylation, are involved in the regulation of proliferation of neural stem cells, differentiation and maturation of neurons, growth of neuronal processes, regulation of dendritic spine density, formation of synapses, and synaptic plasticity [96, 146]. Experimental studies have demonstrated that DNA methylation and histone modifications in the hippocampus synchronously regulate gene transcription during memory formation and storage [147, 148]. The studies in animal models of neurological disorders have revealed the efficacy of inhibitors of histone modifications in the recovery of impaired synaptic plasticity [96].
Dysregulation of epigenetic mechanisms is involved in the development of various pathological conditions, ranging from stress and depression to severe neurodegenerative diseases (NDDs) [149]. Persistent epigenetic changes can be caused by the short-term and long-term stress, suggesting that environmental factors can influence neuronal plasticity by modulating epigenetic regulation in the brain. It was found that chronic stress causes reduction in the length and branching of apical dendrites, as well as dysfunction and decrease in the number of synapses on dendritic spines of pyramidal neurons in the medial prefrontal cortex, neurons in the CA3 region of the hippocampus, and granular neurons of the dentate gyrus [150, 151]. Recently, it was demonstrated that epigenetic modifications are particularly susceptible to the effects of stressors during intrauterine and neonatal development [152]. In confirmation of this, experiments in pregnant female mice have shown that maternal stress induces upregulated expression of DNMT1 and DNMT3A in fetal GABAergic interneurons at the embryonic stages of development, causing further schizophrenia-like behavior in the offspring [153]. It was also found that the intensity of mother-pup contacts during the first week of life in newborn rats changed the status of DNA methylation in the promotor region of the glucocorticoid receptor gene in the offspring brain, which led to a change in the response to stress in adult offspring [154].
In addition to the influence of maternal mental stress during pregnancy, the development of the offspring nervous system and the following brain functioning can be affected by environmental stress factors, such as maternal malnutrition, drug and toxin exposure, alcohol consumption, which can alter the epigenetic status in the offspring brain [155]. Maternal HHcy can be considered as a type of metabolic stress causing long-term adverse effects in the developing fetus, primarily, its nervous system [4]. Our studies demonstrated that experimental maternal HHcy impaired the development of the fetal nervous system and resulted in the decreased resistance of brain cells of the neonates to oxidative stress, leading later to changes in the hypothalamic regulation of the reproductive cycle [156] and suppression of cognitive functions in adult animals [12, 26]. The consequences and the neurotoxic effects of maternal HHcy were studied in details in rat pups in the postnatal period. Maternal HHcy caused a decrease in the number of NeuN-positive neurons and increased the number of GFAP-positive astrocytes and Iba-1-positive microglial cells, as well as promoted p38 MAPK phosphorylation in the parietal cortex and hippocampus of the offspring in the early postnatal development [13, 14]. Moreover, the level and activity of caspase-3 were elevated in the pup cerebral cortex [14]. Maternal HHcy also negatively affected offspring neurogenesis and synaptogenesis, which was manifested as inhibition of proliferation and differentiation of neural stem cells in the hippocampus and decrease in the number of cortical neurons [157], impaired migration of neurons, decreased levels of semaphorin 3E (SEMA3E) and neuronal cell adhesion molecules (NCAMs) involved in this process [5, 23], and decreased contents of synaptophysin and mature BDNF [23, 157]. The study on the structural and ultrastructural organization of the cortex and hippocampus at the early stages of postnatal development in the rat pups that experienced HHcy in prenatal period revealed various degenerative changes in neurons, including organelle lysis in the cytoplasm, destruction of mitochondria, and accumulation of lysosomes, as well as appearance of a large number of glial cells [13, 14]. Furthermore, comparative analysis of changes in the hippocampus and cortex demonstrated the involvement of various neuronal cell death mechanisms due to the specific features of development of these brain regions, which ultimately led to the differences in their susceptibility to prenatal death. Prenatal HHcy caused the development of neuroinflammatory processes in the cortex and hippocampus of rats already at the early stages of postnatal development, which may be a prerequisite for the formation of pronounced cognitive deficits observed in the later period of postnatal development in sexually mature offspring [13, 14]. The hippocampal structures were found to be more vulnerable to the action of Hcy than the cortical structures during brain development [158, 159]. Both short-term and long-term memory appear to be impaired by the HHcy [160]. We found that the impairment of the short-term and long-term memory in the offspring of rats with prenatal HHcy was accompanied by a decrease in the monoaminergic transmission in the hippocampus [26]. The offspring of rats with maternal HHcy demonstrated depletion of catecholamines in the adrenal gland and increase in the blood levels of norepinephrine and epinephrine, which may be the cause of anxiety and increase in the number of errors during learning [26, 161]. Changes in neurotransmission in the brain, primarily in the hippocampus, can cause the impairment of learning and memory performances, as we found using the 8-arm maze test, new object recognition test [26], and Morris water maze test [12]. We also showed that maternal HHcy may have long-term consequences in the postnatal period, leading not only to offspring cognitive deficits, but also to the impaired regulation of reproductive function. The affected offspring female rats demonstrated disturbance in the hypothalamic regulation of reproductive cycles, specifically, the processes associated with the formation of preovulatory peak of gonadotropin-releasing hormone [156]. The effects of prenatal HHcy on the hypothalamic neurotransmitter systems involved in the regulation of reproductive system in female rats were not caused by the increase in the Hcy level in the blood of the offspring in the early postnatal period, but were rather due to the neurotoxic effects of Hcy on the CNS formation during intrauterine development.
The studies of synaptic plasticity in adult animals have revealed that HHcy affects the long-term potentiation (LTP); however, its precise effect depends on the Hcy concentration and duration of exposure [162-166]. The offspring in the prenatal HHcy model also demonstrated decreased LTP [38]. Investigating the role of Hcy in the development of Alzheimer’s disease (AD) showed that in adult animals HHcy decreased the number of hippocampal neurons and their dendrites, as well as the total number of dendritic spines and the number of mushroom spines [166-169]. Similar changes were observed for synaptopodin-positive dendritic spines in a model of prenatal HHcy [38]. Beside altering morphology of neuronal processes, chronic HHcy can influence synaptic transmission through changes in the expression of pre- and postsynaptic proteins. Thus, HHcy decreased the content of presynaptic proteins, such as synaptophysin, synapsin I, synaptotagmin, microtubule-associated protein 2 (MAP-2), and postsynaptic proteins, including the postsynaptic density protein 95 (PSD95), synapse-associated protein 97 (SAP-97) [166-172], and NMDA receptor subunits NR1 and NR2A, in the hippocampus of adult animals [166, 167]. In the maternal HHcy model, the offspring of various age demonstrated decreased expression of the NR1 subunit mRNA [38] and protein [32]. One of the Hcy effects on the mechanisms of neuronal plasticity is modification of neuronal proteins. In cultured hippocampal neuronal cells, folate deficiency, which leads to the increase in the Hcy levels, upregulated the processes of homocysteinylation and aggregation of the motor proteins dynein and kinesin required for the synapse formation and development of neurons [173]. Multiple studies have reported that Hcy induces tau protein hyperphosphorylation and β-amyloid aggregation in hippocampus, which are the cause of cognitive impairments and NDDs associated with a decrease in synaptic plasticity [166-169].
Impairments caused by Hcy at the cellular level can result in severe clinical manifestations. Interestingly, substantia nigra, hippocampus, cerebral cortex, and cerebellum, i.e., the major brain structures involved in the pathogenesis of Parkinson’s diseases, AD, and fetal alcohol syndrome, are especially vulnerable to the neurotoxic effects of Hcy [174-176]. Changes in the Hcy level during pregnancy are an important factor that programs expression of key genes regulating neuronal plasticity in the brain of the fetus and offspring. An increase in the content of methionine (Hcy precursor) in mother’s blood is associated with the development in the offspring of behavioral phenotype similar to schizophrenia and other psychoneurological disorders [20, 177]. An elevated level of Hcy in adults is generally considered as a risk factor of AD development [178]; some studies suggest that prenatal HHcy promotes the development of AD in adult offspring [179, 180].
Using currently available data, Gulyaeva et al. suggested a similarity between the principal molecular mechanisms involved in normal neuroplasticity and neuropathology. Changes in neural plasticity in neuropathology do not ensure the disappearance of neuroplasticity, but rather correspond to changes in the form of this plasticity [181, 182]. This is especially evident in early ontogenesis, when the brain is particularly sensitive to the effects of neurotoxic factors. Depending on the strength and intensity of these effects, changes in the forms of neuroplasticity can lead to the predominant manifestation of the adaptive properties of the nervous system or cause the emergence of prerequisites for the development of neurodegenerative processes both short or long term.
Significant progress has been made in correlating aberrant processes of epigenetic control observed in NDDs with the defects in the development of nervous system leading to respective malformations and functional deficits. While individual epigenetic modifications are not always accompanied by phenotypical changes, their accumulation can lead to such changes. Under continuous exposure, the sensitivity of brain systems to epigenetic modifications increases over time, which elevates the possibility of subsequent epigenetic changes resulting in the disease phenotype. It has been established that dysregulation of brain epigenome is associated with a number of neurological dysfunctions; aberrant DNA methylation and histone modifications are observed in schizophrenia, autism, post-traumatic stress disorder, and AD [183]. Currently, there is a clear understanding that brain development is a lifelong process determined by both genetic and environmental factors. While the genetic code is invariant, epigenetic changes that occur during critical periods and are caused by impaired DNA methylation and histone modifications, can lead to the deterioration of brain plasticity and increase the risk of cognitive decline in later life. Although critical periods are mainly associated with early brain development, they occur throughout the lifetime and include the periods of gamete formation, intrauterine development, puberty, and reproductive aging. All these transitional periods are characterized by the hypersensitivity to the effects of adverse factors on the reproductive function [183].
Studies using various approaches to epigenome editing can contribute to further identification of mechanistic relationship between dysfunctional epigenetic mechanisms and defects in the nervous system development [184]. Since changes in epigenomic parameters can be influenced by environmental factors, future studies should focus on elucidation of how negative environmental impacts lead to particular epigenomic changes and thereby contribute to the pathophysiology of NDDs. The use of epigenetic changes as diagnostic tools can ensure early and correct diagnosis of NDDs, which is of great importance, because developing brain is able to adapt to external stimuli during embryogenesis and early postnatal life [185], so this can be used to prevent certain disorders.
In some cases, a delay in the development of nervous system and/or psychiatric disorders, including those manifested at various life stages (severe forms of depression, schizophrenia, AD, and others) result from impairments in methylation processes in developing brain due to inherited mutations and epigenetic alterations [186]. Therefore, the use of methyl group donors can be one of the approaches for the treatment of these disorders. For example, SAM, as a universal methyl donor, has been proven effective in the treatment of neuropsychiatric diseases throughout the lifetime, although starting the treatment as early in life as possible increases its therapeutic effects and/or even prevents disease manifestation. There is a strong evidence of SAM efficacy in the treatment of already existing major depressive disorder [186, 187]. SAM is recommended as a second-line therapy option after inadequate patient’s response to the treatment with conventional antidepressants. Due to its favorable safety profile, SAM is particularly suitable for the treatment of depression in children, adolescents, and pregnant women. However, further research is needed to approve its use in these potentially vulnerable populations. Recent studies have been focused on SAM as a potential drug in the treatment of AD, a progressive irreversible disease with no effective treatment [188]. A number of risk factors aggravate AD, including deficiency of folic acid and vitamin B12. SAM was found to prevent progression of AD symptoms caused by deficiency in these vitamins. It reduced production of β-amyloid and prevented accumulation of phosphorylated tau protein in the animal models, as well as ameliorated clinical manifestations of AD. Recent clinical trials have shown that SAM can be used as a dietary supplement to maintain or improve the cognitive functions and mood/behavior in AD patients [188]. Despite the fact that SAM has a favorable safety profile, it should still be remembered that it can increase the Hcy levels associated with the risk of developing cardiovascular diseases [186].
Epigenetic modifications are dynamic and reversible. Using inhibitors of these regulatory mechanisms may help to avoid epigenetically evoked alterations in the brain development. Indeed, some of these inhibitors are already used for pharmacological treatment and many are currently under development. For example, HDAC inhibitors trichostatin A and 4-phenylbutyrate and methyltransferase inhibitor 5-aza-deoxycytidine are used for the treatment of fragile X syndrome (Martin–Bell syndrome), hereditary X-linked disease caused by mutation in the FMR1 (fragile X mental retardation 1) gene required for normal CNS development. Another HDAC inhibitor, valproic acid, is used to reduce the frequency of seizures in patients with epilepsy and Rett syndrome, hereditary psychoneurological disease related to the mutation in the X-linked gene encoding the MeCP2 protein [189]. It was generally believed that therapeutics targeting epigenetic regulators are difficult to test and apply in humans because of their low specificity, as well as importance of epigenetic regulation during development. The main limitation is potential off-target effects that were shown, for example, for inhibitors of histone methyltransferase and HDAC that were found to promote the activation of oncogenes and potentially increase the risk of metastasis [190].
Current research efforts in the treatment of NDDs are aimed at targeting specific sequences in the genome by using the CRISPR/dCas9-based therapeutic strategies. Recently, dCas9 fused to different transcriptional activators was used in the mouse model of Dravet syndrome to recover the haploinsufficiency of the SCN1A gene, which plays an important role in the development of various forms of epilepsy [191]. In another study, the dCas9–SunTag protein scaffold system was used to restore the transcallosal communication between the brain hemispheres by targeted delivery of the C11orf46 repressor protein to the promoter of the SEMA6A gene encoding for semaphorin protein, which normalized the growth and branching of axons in developing transcallosal cortical neurons [192]. More straightforward seems the use of a dCas9-based epigenetic approach for the treatment of fragile X-chromosome syndrome accompanied by mental retardation of varying severity. The underlying cause of this complex disorder is a hypermethylation of the FMR1 gene promoter leading to the loss of expression of fragile X mental retardation protein (FMRP) that is involved in synapse formation and plays a key role in maintaining synaptic plasticity. The recruitment of dCas9–TET fusions to the FMR1 gene promoter resulted in loss of its methylation and almost complete restoration of FMRP expression [193]. Despite the existence of approaches to the treatment of NDDs, prevention of these diseases continues to be a priority. Therefore, future studies should be aimed at identification of relevant molecular signaling cascades and their links to epigenetic modifications in a healthy brain and in NDDs. Such studies should also include analysis of the spatial and temporal dynamics of epigenetic mechanisms and patterns, which will help to identify the mechanisms of NDD emergence and to develop the measures for their prevention and treatment.
Modulation of epigenetic modifications, such as DNA methylation and histone modification, in combination with the available methods of antioxidant and neuroprotective therapy, can potentially stimulate the adaptive response of an organism due to the brain plasticity, which will improve clinical manifestation of neuropsychiatric diseases, predisposition to which is formed during pregnancy under the influence of adverse factors (e.g., HHcy). It was recently found that melatonin, a pineal gland hormone with the neuroprotective effect associated with its pronounced antioxidant activity, contributes to the regulation of epigenetic modifications in various NDDs [194]. In view of this, the use of melatonin seems very promising. In the last decade, several studies have reported that melatonin plays an important role in the regulation of neurogenesis and, therefore, can be used as a potential treatment in neurological and mental disorders associated with impaired development of the nervous system [195, 196]. Melatonin promotes the viability, proliferation, and differentiation of hippocampal neurons in vivo and of neural stem cells in vitro [197-199]. In PC12 cells, luzindole (melatonin receptor antagonist) abolished the effects of melatonin, resulting in the suppression of neurite growth and decrease in the number of mature neurons [200]. In rat midbrain neural stem cells, melatonin promoted dopaminergic differentiation of neurons on day 14 of embryonic development by activating the synthesis of BDNF and glia-derived neurotrophic factor (GDNF) [201]. Moreover, melatonin stimulated production of BDNF and GDNF in cells secreting proinflammatory cytokines (IL-18) and suppressed cytokine-induced inhibition of proliferation and differentiation of neural stem cells [196].
The neuroprotective effects of melatonin against the adverse effects of Hcy in the CNS are related to its antioxidant, anti-inflammatory, and antiapoptotic properties and have been studied in details [202]. Some authors emphasize that melatonin scavenges oxygen radicals produced during HHcy and acts as a hormone regulating metabolism of low-weight thiols [202, 203]. It was demonstrated that chronic administration of melatonin to animals with HHcy caused a decrease in the Hcy blood level down to the normal values, whereas an increase in the Hcy content induced by pinealectomy was prevented by administration of melatonin to pinealectomized animals [202]. In addition to directly affecting the Hcy level, melatonin administration stimulated an increase in the glutathione content in tissues, which can also decrease blood Hcy concentration and protect against Hcy neurotoxicity [202, 203].
We believe that neuroprotection during HHcy is still poorly understood and the studies on a possible use of melatonin as an agent decreasing Hcy neurotoxicity are at the very early stage. Besides melatonin, it would be interesting to study the neuroprotector properties of hydrogen sulfide as an antioxidant and anti-inflammatory compound [204] that can prevent the development of oxidative stress in the brain, as well as dysregulation of neuroplasticity and cognitive functions in HHcy [27, 28, 170]. However, this would require further studies on the biochemical mechanisms of high dose H2S neurotoxic side effects.
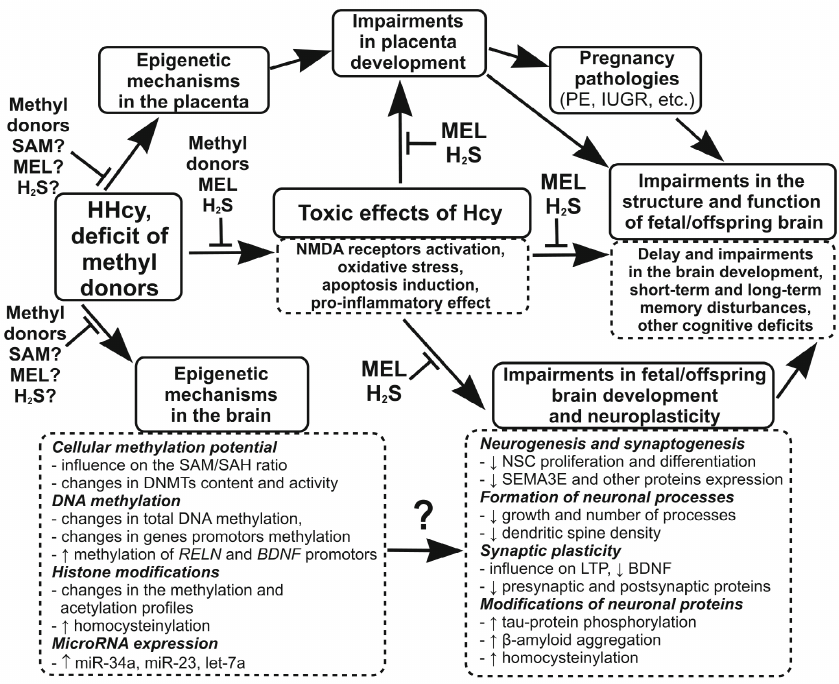
Based on the above data, we suggest that in addition to the relatively well-studied neurotoxic effects of maternal HHcy, such as activation of NMDA receptors, induction of oxidative stress and apoptosis, and development of neuroinflammatory response, HHcy can adversely affect the development of the offspring brain via epigenetic mechanisms, including DNA methylation, post-translational histone modifications, and microRNA expression. A large body of accumulated evidence shows that HHcy impairs neuronal plasticity, in particular LTP, and decreases the levels of pre- and post-synaptic proteins, which may explain the cognitive deficits in the offspring experiencing prenatal HHcy. The data presented in the review allow us to hypothesize that the cause of the HHcy-induced disturbances in the brain plasticity, along with the known neurotoxic effects of Hcy, is its influence on the epigenetic mechanisms controlling expression of genes involved in the regulation of neuroplasticity. Impairments in the placenta formation during maternal HHcy, in particular, in the formation of placental circulatory network, can have an additional negative impact on the fetal brain development by reducing the supply of oxygen, nutrients, and compounds necessary for the normal development of fetal brain. The negative influence of maternal HHcy on the brain development and neuronal plasticity can have consequences for the offspring at the early age (reduces cognitive and mental abilities and predisposition to developing autism), as well as more delayed effects (predisposition to neuropsychiatric diseases in adulthood and NDDs at the old age). Methyl donors (folic acid, vitamin B12, betaine, choline) can be used as universal protectors against the negative effects of HHcy, because they reduce the level of Hcy through activation of its re-methylation to methionine. Compounds with the antioxidant and anti-inflammatory properties (melatonin, H2S, etc.) can also be used as protectors against the toxic effects of Hcy. Beside the mentioned methyl donors that reduce the level of Hcy, SAM and other modulators of epigenetic processes can prevent the influence of HHcy on the epigenetic mechanisms by restoring intracellular methylation potential reduced in HHcy. The figure shows the effects of Hcy on the epigenetic regulation and neuronal plasticity involved in the disorders in the fetal brain development and cognitive deficits in the offspring experiencing maternal HHcy, as well as possible contribution of the placental component and proposed mechanisms of the protective action of neuroprotectors.
The influence of maternal HHcy on the processes of epigenetic regulation and neuronal plasticity, which may be involved in fetal and early postnatal brain development disturbances, as well as in offspring cognitive deficits. HHcy or methyl donor deficiency, which in most cases leads to the increase in the Hcy level, changes intracellular methylation potential (SAM/SAH ratio) and modulates the activity and expression of DNMTs. These processes affect gene expression via epigenetic mechanisms such as DNA methylation and post-translational histone modifications (methylation or acetylation). Hcy is also capable of influencing gene expression through direct binding to histones (homocysteinylation) and, probably, via changes in the microRNA expression. Maternal HHcy can modulate epigenetic mechanisms that control expression of proteins responsible for neurogenesis, gliogenesis, synaptogenesis, and synaptic signal transmission. This may be one of the causes for the impairments in the fetal or neonate brain development that adversely influences the cognitive abilities of the offspring due to the decrease in the neuronal plasticity. HHcy can also disturb the formation of the placenta, in particular, its vascular network, as observed in PE and IUGR. Insufficient blood supply to the placenta, in turn, may lead to the decrease in the supply to the fetus of compounds necessary for the normal fetal brain development. The use of methyl donors (folic acid, vitamin B12, betaine, choline, etc.) as compounds reducing the content of Hcy is effective at the early stages and prevents the negative toxic and epigenetic effects of HHcy. Because of the development of oxidative stress during HHcy, substances with antioxidant and anti-inflammatory properties (melatonin, H2S, etc.) can abolish the toxic effects of Hcy. SAM can protect against the negative epigenetic effects of HHcy associated with the inhibition of DNA methylation because it restores intracellular methylation potential, which is reduced in HHcy. Designations: NSCs, neural stem cells; MEL, melatonin; ↑, increase; ↓, decrease
CONCLUSION
DNA methylation, posttranslational modifications of histones, and microRNA expression are among the most important epigenetic mechanisms. They are involved in the regulation of transcription of genes responsible for brain development, especially in early ontogenesis. It has become evident that epigenetic modifications play an essential role in the regulation of adaptive neuroplasticity. It has been established that Hcy, a component of the one-carbon metabolism, can influence epigenetic processes in the fetal brain and placenta. We suggest that maternal HHcy negatively impacts the development of fetal brain not only by producing the neurotoxic effects, which have been comprehensive studied, but also by modulating epigenetic mechanisms involved in the regulating neuroplasticity, which may be one of reasons for the impaired cognitive abilities in the offspring of mothers with HHcy. Being a risk factor for the development of cognitive deficits in early life, prenatal HHcy can also have long-term consequences, such as development of neuropsychiatric disorders in adulthood and, possibly, a predisposition to NDDs in older age. Despite the advances in studying this topic, many issues related to the molecular mechanisms ensuring the negative effects HHcy on epigenetic regulation, their consequences, and possible defense against such influence require further investigation. Studying the role of epigenetic mechanisms in the regulation of neuronal plasticity in the brain, as well as of the epigenetic effects of Hcy, is of particular interest in connection with the development of strategies for the epigenome modulation, which can be used to prevent or ameliorate various neurological pathologies, for which HHcy is one of the risk factors.
Contributions. A. V. Arutjunyan developed the review concept; A. V. Arutjunyan, G. O. Kerkeshko, Yu. P. Milyutina, A. D. Shcherbitskaia, and I. V. Zalozniaia collected and discussed the published data; A. V. Arutjunyan, G. O. Kerkeshko, Yu. P. Milyutina, A. D. Shcherbitskaia, and I. V. Zalozniaia wrote the manuscript; A. V. Arutjunyan and G. O. Kerkeshko edited the text.
Funding. This study was supported by the Russian Science Foundation (project no. 22-15-00393).
Ethics declarations. The authors declare no conflict of interests. This review does not contain studies involving humans or animal subject performed by any of the authors.
REFERENCES
1.Irvine, N., England-Mason, G., Field, C. J., Dewey,
D., and Aghajafari, F. (2022) Prenatal folate and choline levels and
brain and cognitive development in children: a critical narrative
review, Nutrients, 14, 364, doi: 10.3390/nu14020364.
2.Naninck, E. F. G., Stijger, P. C., and
Brouwer-Brolsma, E. M. (2019) The importance of maternal folate status
for brain development and function of offspring, Adv. Nutr.,
10, 502-519, doi: 10.1093/advances/nmy120.
3.Korsmo, H. W., and Jiang, X. (2021) One carbon
metabolism and early development: a diet-dependent destiny, Trends
Endocrinol. Metab., 32, 579-593, doi:
10.1016/j.tem.2021.05.011.
4.Arutjunyan, A. V., Kerkeshko, G. O., Milyutina, Y.
P., Shcherbitskaia, A. D., and Zalozniaia, I. V. (2021) Prenatal stress
in maternal hyperhomocysteinemia: impairments in the fetal nervous
system development and placental function, Biochemistry
(Moscow), 86, 716-728, doi: 10.1134/S0006297921060092.
5.Baydas, G., Koz, S. T., Tuzcu, M., Nedzvetsky, V.
S., and Etem, E. (2007) Effects of maternal hyperhomocysteinemia
induced by high methionine diet on the learning and memory performance
in offspring, Int. J. Dev. Neurosci., 25, 133-139, doi:
10.1016/j.ijdevneu.2007.03.001.
6.Baydas, G., Koz, S. T., Tuzcu, M., and Nedzvetsky,
V. S. (2008) Melatonin prevents gestational
hyperhomocysteinemia-associated alterations in neurobehavioral
developments in rats, J. Pineal Res., 44, 181-188, doi:
10.1111/j.1600-079X.2007.00506.x.
7.Koz, S. T., Gouwy, N. T., Demir, N., Nedzvetsky, V.
S., Etem, E., and Baydas, G. (2010) Effects of maternal
hyperhomocysteinemia induced by methionine intake on oxidative stress
and apoptosis in pup rat brain, Int. J. Dev. Neurosci.,
28, 325-329, doi: 10.1016/j.ijdevneu.2010.02.006.
8.Pustygina, A. V., Milyutina, Y. P., Zaloznyaya, I.
V., and Arutyunyan, A. V. (2015) Indices of oxidative stress in the
brain of newborn rats subjected to prenatal hyperhomocysteinemia,
Neurochem. J., 9, 60-65, doi:
10.1134/s1819712415010079.
9.Geoffroy, A., Kerek, R., Pourie, G., Helle, D.,
Gueant, J. L., Daval, J. L., and Bossenmeyer-Pourie, C. (2017) Late
maternal folate supplementation rescues from methyl donor
deficiency-associated brain defects by restoring Let-7 and miR-34
pathways, Mol. Neurobiol., 54, 5017-5033, doi:
10.1007/s12035-016-0035-8.
10.Geoffroy, A., Saber-Cherif, L., Pourie, G.,
Helle, D., Umoret, R., Gueant, J. L., Bossenmeyer-Pourie, C., and
Daval, J. L. (2019) Developmental impairments in a rat model of methyl
donor deficiency: effects of a late maternal supplementation with folic
acid, Int. J. Mol. Sci., 20, 973, doi:
10.3390/ijms20040973.
11.Arutjunyan, A. V., Milyutina, Y. P.,
Shcherbitskaia, A. D., Kerkeshko, G. O., Zalozniaia, I. V., and Mikhel,
A. V. (2020) Neurotrophins of the fetal brain and placenta in prenatal
hyperhomocysteinemia, Biochemistry (Moscow), 85, 248-259,
doi: 10.1134/S000629792002008X.
12.Arutjunyan, A., Kozina, L., Stvolinskiy, S.,
Bulygina, Y., Mashkina, A., and Khavinson, V. (2012) Pinealon protects
the rat offspring from prenatal hyperhomocysteinemia, Int. J. Clin.
Exp. Med., 5, 179-185.
13.Shcherbitskaia, A. D., Vasilev, D. S., Milyutina,
Y. P., Tumanova, N. L., Mikhel, A. V., Zalozniaia, I. V., and
Arutjunyan, A. V. (2021) Prenatal hyperhomocysteinemia induces glial
activation and alters neuroinflammatory marker expression in infant rat
hippocampus, Cells, 10, 1536, doi:
10.3390/cells10061536.
14.Shcherbitskaia, A. D., Vasilev, D. S., Milyutina,
Y. P., Tumanova, N. L., Zalozniaia, I. V., Kerkeshko, G. O., and
Arutjunyan, A. V. (2020) Maternal hyperhomocysteinemia induces
neuroinflammation and neuronal death in the rat offspring cortex,
Neurotox Res., 38, 408-420, doi:
10.1007/s12640-020-00233-w.
15.Jadavji, N. M., Deng, L., Malysheva, O., Caudill,
M. A., and Rozen, R. (2015) MTHFR deficiency or reduced intake of
folate or choline in pregnant mice results in impaired short-term
memory and increased apoptosis in the hippocampus of wild-type
offspring, Neuroscience, 300, 1-9, doi:
10.1016/j.neuroscience.2015.04.067.
16.Saber Cherif, L., Pourie, G., Geoffroy, A.,
Julien, A., Helle, D., Robert, A., Umoret, R., Gueant, J. L.,
Bossenmeyer-Pourie, C., and Daval, J. L. (2019) Methyl donor deficiency
during gestation and lactation in the rat affects the expression of
neuropeptides and related receptors in the hypothalamus, Int. J.
Mol. Sci., 20, 5097, doi: 10.3390/ijms20205097.
17.Schweinberger, B. M., Rodrigues, A. F., Turcatel,
E., Pierozan, P., Pettenuzzo, L. F., Grings, M., Scaini, G., Parisi, M.
M., Leipnitz, G., Streck, E. L., Barbe-Tuana, F. M., and Wyse, A. T. S.
(2018) Maternal hypermethioninemia affects neurons number,
neurotrophins levels, energy metabolism, and
Na+,K+-ATPase expression/content in brain of rat
offspring, Mol. Neurobiol., 55, 980-988, doi:
10.1007/s12035-017-0383-z.
18.De Crescenzo, A. H., Panoutsopoulos, A. A., Tat,
L., Schaaf, Z., Racherla, S., Henderson, L., Leung, K. Y., Greene, N.
D. E., Green, R., and Zarbalis, K. S. (2020) Deficient or excess folic
acid supply during pregnancy alter cortical neurodevelopment in mouse
offspring, Cereb. Cortex, 31, 635-649, doi:
10.1093/cercor/bhaa248.
19.Li, W., Li, Z., Zhou, D., Zhang, X., Yan, J., and
Huang, G. (2019) Maternal folic acid deficiency stimulates neural cell
apoptosis via miR-34a associated with Bcl-2 in the rat foetal brain,
Int. J. Dev. Neurosci., 72, 6-12, doi:
10.1016/j.ijdevneu.2018.11.002.
20.Chen, S., Alhassen, W., Yoshimura, R., De Silva,
A., Abbott, G. W., Baldi, P., and Alachkar, A. (2020) Metabolomic and
transcriptomic signatures of prenatal excessive methionine support
nature rather than nurture in schizophrenia pathogenesis, Commun.
Biol., 3, 409, doi: 10.1038/s42003-020-01124-8.
21.Craciunescu, C. N., Johnson, A. R., and Zeisel,
S. H. (2010) Dietary choline reverses some, but not all, effects of
folate deficiency on neurogenesis and apoptosis in fetal mouse brain,
J. Nutr., 140, 1162-1166, doi: 10.3945/jn.110.122044.
22.Milyutina, Yu. P., Shcherbitskaya, A. D.,
Saltykova, E. D., Kozina, L. S., Zhuravin, I. A., Nalivaeva, N. N., and
Arutjunyan, A. V. (2017) Metabolic impairments in the placenta and
brain of the fetuses of pregnant rats after experimental
hyperhomocysteinemia, Ross. Physiol. Zhurn. im. I. M. Sechenova,
103, 1280-1291.
23.Vasilev, D. S., Shcherbitskaia, A. D., Tumanova,
N. L., Mikhel, A. V., Milyutina, Y. P., Kovalenko, A. A., Dubrovskaya,
N. M., Inozemtseva, D. B., Zalozniaia, I. V., and Arutjunyan, A. V.
(2023) Maternal hyperhomocysteinemia disturbs the mechanisms of
embryonic brain development and its maturation in early postnatal
ontogenesis, Cells, 12, 189, doi:
10.3390/cells12010189.
24.Blaise, S. A., Nedelec, E., Schroeder, H.,
Alberto, J. M., Bossenmeyer-Pourie, C., Gueant, J. L., and Daval, J. L.
(2007) Gestational vitamin B deficiency leads to
homocysteine-associated brain apoptosis and alters neurobehavioral
development in rats, Am. J. Pathol., 170, 667-679, doi:
10.2353/ajpath.2007.060339.
25.Blaise, S. A., Nedelec, E., Alberto, J. M.,
Schroeder, H., Audonnet, S., Bossenmeyer-Pourie, C., Gueant, J. L., and
Daval, J. L. (2009) Short hypoxia could attenuate the adverse effects
of hyperhomocysteinemia on the developing rat brain by inducing
neurogenesis, Exp. Neurol., 216, 231-238, doi:
10.1016/j.expneurol.2008.11.020.
26.Shcherbitskaya, A. D., Milyutina, Y. P.,
Zaloznyaya, I. V., Arutjunyan, A. V., Nalivaeva, N. N., and Zhuravin,
I. A. (2017) The effects of prenatal hyperhomocysteinemia on the
formation of memory and the contents of biogenic amines in the rat
hippocampus, Neurochem. J., 11, 296-301, doi:
10.1134/s1819712417040080.
27.Yakovleva, O. V., Ziganshina, A. R., Dmitrieva,
S. A., Arslanova, A. N., Yakovlev, A. V., Minibayeva, F. V.,
Khaertdinov, N. N., Ziyatdinova, G. K., Giniatullin, R. A., and
Sitdikova, G. F. (2018) Hydrogen sulfide ameliorates developmental
impairments of rat offspring with prenatal hyperhomocysteinemia,
Oxid. Med. Cell. Longev., 2018, 2746873, doi:
10.1155/2018/2746873.
28.Yakovleva, O., Bogatova, K., Mukhtarova, R.,
Yakovlev, A., Shakhmatova, V., Gerasimova, E., Ziyatdinova, G.,
Hermann, A., and Sitdikova, G. (2020) Hydrogen sulfide alleviates
anxiety, motor, and cognitive dysfunctions in rats with maternal
hyperhomocysteinemia via mitigation of oxidative stress,
Biomolecules, 10, 995, doi: 10.3390/biom10070995.
29.Gerasimova, E., Burkhanova, G., Chernova, K.,
Zakharov, A., Enikeev, D., Khaertdinov, N., Giniatullin, R., and
Sitdikova, G. (2021) Hyperhomocysteinemia increases susceptibility to
cortical spreading depression associated with photophobia, mechanical
allodynia, and anxiety in rats, Behav. Brain Res., 409,
113324, doi: 10.1016/j.bbr.2021.113324.
30.Gerasimova, E., Yakovleva, O., Enikeev, D.,
Bogatova, K., Hermann, A., Giniatullin, R., and Sitdikova, G. (2022)
Hyperhomocysteinemia increases cortical excitability and aggravates
mechanical hyperalgesia and anxiety in a nitroglycerine-induced
migraine model in rats, Biomolecules, 12, 735, doi:
10.3390/biom12050735.
31.Hassan, Z., Coelho, D., Kokten, T., Alberto, J.
M., Umoret, R., Daval, J. L., Gueant, J. L., Bossenmeyer-Pourie, C.,
and Pourie, G. (2019) Brain susceptibility to methyl donor deficiency:
from fetal programming to aging outcome in rats, Int. J. Mol.
Sci., 20, 5692, doi: 10.3390/ijms20225692.
32.Pourie, G., Martin, N., Daval, J. L., Alberto, J.
M., Umoret, R., Gueant, J. L., and Bossenmeyer-Pourie, C. (2020) The
stimulation of neurogenesis improves the cognitive status of aging rats
subjected to gestational and perinatal deficiency of B9-12 vitamins,
Int. J. Mol. Sci., 21, 8008, doi:
10.3390/ijms21218008.
33.Figueiro, P.W., de Moreira, D. S., Dos Santos, T.
M., Prezzi, C. A., Rohden, F., Faccioni-Heuser, M. C., Manfredini, V.,
Netto, C. A., and Wyse, A. T. S. (2019) The neuroprotective role of
melatonin in a gestational hypermethioninemia model, Int. J. Dev.
Neurosci., 78, 198-209, doi:
10.1016/j.ijdevneu.2019.08.004.
34.Schweinberger, B. M., Rodrigues, A. F., Dos
Santos, T. M., Rohden, F., Barbosa, S., da Luz Soster, P. R., Partata,
W. A., Faccioni-Heuser, M. C., and Wyse, A. T. S. (2018) Methionine
administration in pregnant rats causes memory deficit in the offspring
and alters ultrastructure in brain tissue, Neurotox. Res.,
33, 239-246, doi: 10.1007/s12640-017-9830-x.
35.Zhou, D., Li, Z., Sun, Y., Yan, J., Huang, G.,
and Li, W. (2022) Early life stage folic acid deficiency delays the
neurobehavioral development and cognitive function of rat offspring by
hindering de novo telomere synthesis, Int. J. Mol. Sci.,
23, 6948, doi: 10.3390/ijms23136948.
36.Degroote, S., Hunting, D., and Takser, L. (2018)
Periconceptional folate deficiency leads to autism-like traits in
Wistar rat offspring, Neurotoxicol. Teratol., 66,
132-138, doi: 10.1016/j.ntt.2017.12.008.
37.Makhro, A. V., Mashkina, A. P., Solenaya, O. A.,
Trunova, O. A., Kozina, L. S., Arutyunian, A. V., and Bulygina, E. R.
(2008) Prenatal hyperhomocysteinemia as a model of oxidative stress of
the brain, Bull. Exp. Biol. Med., 146, 33-35, doi:
10.1007/s10517-008-0233-0.
38.Postnikova, T. Y., Amakhin, D. V., Trofimova, A.
M., Tumanova, N. L., Dubrovskaya, N. M., Kalinina, D. S., Kovalenko, A.
A., Shcherbitskaia, A. D., Vasilev, D. S., and Zaitsev, A. V. (2022)
Maternal hyperhomocysteinemia produces memory deficits associated with
impairment of long-term synaptic plasticity in young rats,
Cells, 12, 58, doi: 10.3390/cells12010058.
39.Imbard, A., Benoist, J. F., and Blom, H. J.
(2013) Neural tube defects, folic acid and methylation, Int. J.
Environ. Res. Public Health, 10, 4352-4389, doi:
10.3390/ijerph10094352.
40.Tang, K. F., Li, Y. L., and Wang, H. Y. (2015)
Quantitative assessment of maternal biomarkers related to one-carbon
metabolism and neural tube defects, Sci. Rep., 5, 8510,
doi: 10.1038/srep08510.
41.Murphy, M. M., Fernandez-Ballart, J. D., Molloy,
A. M., and Canals, J. (2017) Moderately elevated maternal homocysteine
at preconception is inversely associated with cognitive performance in
children 4 months and 6 years after birth, Matern. Child Nutr.,
13, e12289, doi: 10.1111/mcn.12289.
42.Campoy, C., Azaryah, H., Torres-Espinola, F. J.,
Martinez-Zaldivar, C., Garcia-Santos, J. A., Demmelmair, H., Haile, G.,
Gyorei, E., Ramirez-Tortosa, M. D. C., Reischl, E., Rzehak, P., Molloy,
A. M., Decsi, T., Luna, J. D., Koletzko, B., and Perez-Garcia, M.
(2020) Long-chain polyunsaturated fatty acids, homocysteine at birth
and fatty acid desaturase gene cluster polymorphisms are associated
with Children’s processing speed up to age 9 years,
Nutrients, 13, 131, doi: 10.3390/nu13010131.
43.Srinivasan, K., Thomas, T., Kapanee, A. R.,
Ramthal, A., Bellinger, D. C., Bosch, R. J., Kurpad, A. V., and Duggan,
C. (2017) Effects of maternal vitamin B12 supplementation on
early infant neurocognitive outcomes: a randomized controlled clinical
trial, Matern. Child Nutr., 13, e12325, doi:
10.1111/mcn.12325.
44.Ars, C. L., Nijs, I. M., Marroun, H. E., Muetzel,
R., Schmidt, M., Steenweg-de Graaff, J., van der Lugt, A., Jaddoe, V.
W., Hofman, A., Steegers, E. A., Verhulst, F. C., Tiemeier, H., and
White, T. (2019) Prenatal folate, homocysteine and vitamin
B12 levels and child brain volumes, cognitive development
and psychological functioning: the generation R study, Br. J.
Nutr., 122, S1-S9, doi: 10.1017/S0007114515002081.
45.Boldyrev, A. A. (2009) Molecular mechanisms of
homocysteine toxicity, Biochemistry (Moscow), 74,
589-598, doi: 10.1134/s0006297909060017.
46.Troen, A. M. (2005) The central nervous system in
animal models of hyperhomocysteinemia, Prog. Neuropsychopharmacol.
Biol. Psychiatry, 29, 1140-1151, doi:
10.1016/j.pnpbp.2005.06.025.
47.Esse, R., Barroso, M., Tavares de Almeida, I.,
and Castro, R. (2019) The contribution of homocysteine metabolism
disruption to endothelial dysfunction: state-of-the-art, Int. J.
Mol. Sci., 20, 867, doi: 10.3390/ijms20040867.
48.Faverzani, J. L., Hammerschmidt, T. G., Sitta,
A., Deon, M., Wajner, M., and Vargas, C. R. (2017) Oxidative stress in
homocystinuria due to cystathionine ss-synthase deficiency: findings in
patients and in animal models, Cell. Mol. Neurobiol., 37,
1477-1485, doi: 10.1007/s10571-017-0478-0.
49.Lehotsky, J., Tothova, B., Kovalska, M., Dobrota,
D., Benova, A., Kalenska, D., and Kaplan, P. (2016) Role of
homocysteine in the ischemic stroke and development of ischemic
tolerance, Front. Neurosci., 10, 538, doi:
10.3389/fnins.2016.00538.
50.Sibarov, D. A., Abushik, P. A., Giniatullin, R.,
and Antonov, S. M. (2016) GluN2A subunit-containing NMDA receptors are
the preferential neuronal targets of homocysteine, Front. Cell.
Neurosci., 10, 246, doi: 10.3389/fncel.2016.00246.
51.Poddar, R. (2021) Hyperhomocysteinemia is an
emerging comorbidity in ischemic stroke, Exp. Neurol.,
336, 113541, doi: 10.1016/j.expneurol.2020.113541.
52.Sharma, G. S., Kumar, T., Dar, T. A., and Singh,
L. R. (2015) Protein N-homocysteinylation: From cellular toxicity to
neurodegeneration, Biochim. Biophys. Acta, 1850,
2239-2245, doi: 10.1016/j.bbagen.2015.08.013.
53.Jakubowski, H. (2019) Homocysteine modification
in protein structure/function and human disease, Physiol. Rev.,
99, 555-604, doi: 10.1152/physrev.00003.2018.
54.Selhub, J. (1999) Homocysteine metabolism,
Annu. Rev. Nutr., 19, 217-246, doi:
10.1146/annurev.nutr.19.1.217.
55.Gueant, J. L., Namour, F., Gueant-Rodriguez, R.
M., and Daval, J. L. (2013) Folate and fetal programming: a play in
epigenomics? Trends Endocrinol. Metab., 24, 279-289, doi:
10.1016/j.tem.2013.01.010.
56.James, S. J., Melnyk, S., Pogribna, M., Pogribny,
I. P., and Caudill, M. A. (2002) Elevation in S-adenosylhomocysteine
and DNA hypomethylation: potential epigenetic mechanism for
homocysteine-related pathology, J. Nutr., 132,
2361S-2366S, doi: 10.1093/jn/132.8.2361S.
57.Hannibal, L., and Blom, H. J. (2017) Homocysteine
and disease: causal associations or epiphenomenons? Mol. Aspects
Med., 53, 36-42, doi: 10.1016/j.mam.2016.11.003.
58.Dayal, S., and Lentz, S. R. (2008) Murine models
of hyperhomocysteinemia and their vascular phenotypes, Arterioscler.
Thromb. Vasc. Biol., 28, 1596-1605, doi:
10.1161/ATVBAHA.108.166421.
59.McGee, M., Bainbridge, S., and Fontaine-Bisson,
B. (2018) A crucial role for maternal dietary methyl donor intake in
epigenetic programming and fetal growth outcomes, Nutr. Rev.,
76, 469-478, doi: 10.1093/nutrit/nuy006.
60.Heil, S. G., Herzog, E. M., Griffioen, P. H., van
Zelst, B., Willemsen, S. P., de Rijke, Y. B., Steegers-Theunissen, R.
P. M., and Steegers, E. A. P. (2019) Lower S-adenosylmethionine levels
and DNA hypomethylation of placental growth factor (PlGF) in placental
tissue of early-onset preeclampsia-complicated pregnancies, PLoS
One, 14, e0226969, doi: 10.1371/journal.pone.0226969.
61.Lin, N., Qin, S., Luo, S., Cui, S., Huang, G.,
and Zhang, X. (2014) Homocysteine induces cytotoxicity and
proliferation inhibition in neural stem cells via DNA methylation in
vitro, FEBS J., 281, 2088-2096, doi:
10.1111/febs.12764.
62.Perla-Kajan, J., and Jakubowski, H. (2019)
Dysregulation of epigenetic mechanisms of gene expression in the
pathologies of hyperhomocysteinemia, Int. J. Mol. Sci.,
20, 3140, doi: 10.3390/ijms20133140.
63.Kim, K. C., Friso, S., and Choi, S. W. (2009) DNA
methylation, an epigenetic mechanism connecting folate to healthy
embryonic development and aging, J. Nutr. Biochem., 20,
917-926, doi: 10.1016/j.jnutbio.2009.06.008.
64.Bhutani, N., Burns, D. M., and Blau, H. M. (2011)
DNA demethylation dynamics, Cell, 146, 866-872, doi:
10.1016/j.cell.2011.08.042.
65.Freitag, M., and Selker, E. U. (2005) Controlling
DNA methylation: many roads to one modification, Curr. Opin. Genet.
Dev., 15, 191-199, doi: 10.1016/j.gde.2005.02.003.
66.Weber, M., Hellmann, I., Stadler, M. B., Ramos,
L., Paabo, S., Rebhan, M., and Schubeler, D. (2007) Distribution,
silencing potential and evolutionary impact of promoter DNA methylation
in the human genome, Nat. Genet., 39, 457-466, doi:
10.1038/ng1990.
67.Jaenisch, R., and Bird, A. (2003) Epigenetic
regulation of gene expression: how the genome integrates intrinsic and
environmental signals, Nat. Genet., 33 Suppl., 245-254,
doi: 10.1038/ng1089.
68.Mandaviya, P. R., Stolk, L., and Heil, S. G.
(2014) Homocysteine and DNA methylation: a review of animal and human
literature, Mol. Genet. Metab., 113, 243-252, doi:
10.1016/j.ymgme.2014.10.006.
69.Ito, S., D’Alessio, A. C., Taranova, O. V.,
Hong, K., Sowers, L. C., and Zhang, Y. (2010) Role of Tet proteins in
5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass
specification, Nature, 466, 1129-1133, doi:
10.1038/nature09303.
70.Tahiliani, M., Koh, K. P., Shen, Y., Pastor, W.
A., Bandukwala, H., Brudno, Y., Agarwal, S., Iyer, L.M., Liu, D. R.,
Aravind, L., and Rao, A. (2009) Conversion of 5-methylcytosine to
5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1,
Science, 324, 930-935, doi: 10.1126/science.1170116.
71.Globisch, D., Munzel, M., Muller, M., Michalakis,
S., Wagner, M., Koch, S., Bruckl, T., Biel, M., and Carell, T. (2010)
Tissue distribution of 5-hydroxymethylcytosine and search for active
demethylation intermediates, PLoS One, 5, e15367, doi:
10.1371/journal.pone.0015367.
72.Song, C. X., Szulwach, K. E., Fu, Y., Dai, Q.,
Yi, C., Li, X., Li, Y., Chen, C. H., Zhang, W., Jian, X., Wang, J.,
Zhang, L., Looney, T. J., Zhang, B., Godley, L. A., Hicks, L. M., Lahn,
B. T., Jin, P., and He, C. (2011) Selective chemical labeling reveals
the genome-wide distribution of 5-hydroxymethylcytosine, Nat.
Biotechnol., 29, 68-72, doi: 10.1038/nbt.1732.
73.Szulwach, K. E., Li, X., Li, Y., Song, C. X., Wu,
H., Dai, Q., Irier, H., Upadhyay, A. K., Gearing, M., Levey, A. I.,
Vasanthakumar, A., Godley, L. A., Chang, Q., Cheng, X., He, C., and
Jin, P. (2011) 5-hmC-mediated epigenetic dynamics during postnatal
neurodevelopment and aging, Nat. Neurosci., 14,
1607-1616, doi: 10.1038/nn.2959.
74.Scott, J. M., Molloy, A. M., Kennedy, D. G.,
Kennedy, S., and Weir, D. G. (1994) Effects of the disruption of
transmethylation in the central nervous system: an animal model,
Acta Neurol. Scand. Suppl., 154, 27-31, doi:
10.1111/j.1600-0404.1994.tb05406.x.
75.Schatz, R. A., Wilens, T. E., and Sellinger, O.
Z. (1981) Decreased transmethylation of biogenic amines after in
vivo elevation of brain S-adenosyl-l-homocysteine, J.
Neurochem., 36, 1739-1748, doi:
10.1111/j.1471-4159.1981.tb00426.x.
76.Esse, R., Imbard, A., Florindo, C., Gupta, S.,
Quinlivan, E.P., Davids, M., Teerlink, T., Tavares de Almeida, I.,
Kruger, W. D., Blom, H. J., and Castro, R. (2014) Protein arginine
hypomethylation in a mouse model of cystathionine beta-synthase
deficiency, FASEB J., 28, 2686-2695, doi:
10.1096/fj.13-246579.
77.Lee, H. O., Wang, L., Kuo, Y. M., Gupta, S.,
Slifker, M. J., Li, Y. S., Andrews, A. J., and Kruger, W. D. (2017)
Lack of global epigenetic methylation defects in CBS deficient mice,
J. Inherit. Metab. Dis., 40, 113-120, doi:
10.1007/s10545-016-9958-5.
78.DiBello, P. M., Dayal, S., Kaveti, S., Zhang, D.,
Kinter, M., Lentz, S. R., and Jacobsen, D. W. (2010) The nutrigenetics
of hyperhomocysteinemia: quantitative proteomics reveals differences in
the methionine cycle enzymes of gene-induced versus diet-induced
hyperhomocysteinemia, Mol. Cell. Proteomics, 9, 471-485,
doi: 10.1074/mcp.M900406-MCP200.
79.Suszynska-Zajczyk, J., and Jakubowski, H. (2014)
Paraoxonase 1 and dietary hyperhomocysteinemia modulate the expression
of mouse proteins involved in liver homeostasis, Acta Biochim.
Pol., 61, 815-823.
80.Li, J. G., Barrero, C., Gupta, S., Kruger, W. D.,
Merali, S., and Pratico, D. (2017) Homocysteine modulates
5-lipoxygenase expression level via DNA methylation, Aging Cell,
16, 273-280, doi: 10.1111/acel.12550.
81.Pogribny, I. P., Karpf, A. R., James, S. R.,
Melnyk, S., Han, T., and Tryndyak, V. P. (2008) Epigenetic alterations
in the brains of Fisher 344 rats induced by long-term administration of
folate/methyl-deficient diet, Brain Res., 1237, 25-34,
doi: 10.1016/j.brainres.2008.07.077.
82.Kalani, A., Kamat, P. K., Familtseva, A.,
Chaturvedi, P., Muradashvili, N., Narayanan, N., Tyagi, S. C., and
Tyagi, N. (2014) Role of microRNA29b in blood-brain barrier dysfunction
during hyperhomocysteinemia: an epigenetic mechanism, J. Cereb.
Blood Flow Metab., 34, 1212-1222, doi:
10.1038/jcbfm.2014.74.
83.Kalani, A., Kamat, P. K., Givvimani, S., Brown,
K., Metreveli, N., Tyagi, S. C., and Tyagi, N. (2014) Nutri-epigenetics
ameliorates blood-brain barrier damage and neurodegeneration in
hyperhomocysteinemia: role of folic acid, J. Mol. Neurosci.,
52, 202-215, doi: 10.1007/s12031-013-0122-5.
84.Fan, G., Beard, C., Chen, R. Z., Csankovszki, G.,
Sun, Y., Siniaia, M., Biniszkiewicz, D., Bates, B., Lee, P. P., Kuhn,
R., Trumpp, A., Poon, C., Wilson, C. B., and Jaenisch, R. (2001) DNA
hypomethylation perturbs the function and survival of CNS neurons in
postnatal animals, J. Neurosci., 21, 788-797, doi:
10.1523/JNEUROSCI.21-03-00788.2001.
85.Hutnick, L. K., Golshani, P., Namihira, M., Xue,
Z., Matynia, A., Yang, X. W., Silva, A. J., Schweizer, F. E., and Fan,
G. (2009) DNA hypomethylation restricted to the murine forebrain
induces cortical degeneration and impairs postnatal neuronal
maturation, Hum. Mol. Genet., 18, 2875-2888, doi:
10.1093/hmg/ddp222.
86.Liu, H. Y., Liu, S. M., and Zhang, Y. Z. (2020)
Maternal folic acid supplementation mediates offspring health via DNA
methylation, Reprod. Sci., 27, 963-976, doi:
10.1007/s43032-020-00161-2.
87.Li, W., Li, Z., Li, S., Wang, X., Wilson, J. X.,
and Huang, G. (2018) Periconceptional folic acid supplementation
benefit to development of early sensory-motor function through increase
DNA methylation in rat offspring, Nutrients, 10, 292,
doi: 10.3390/nu10030292.
88.Wang, X., Li, Z., Zhu, Y., Yan, J., Liu, H.,
Huang, G., and Li, W. (2021) Maternal folic acid impacts DNA
methylation profile in male rat offspring implicated in
neurodevelopment and learning/memory abilities, Genes Nutr.,
16, 1, doi: 10.1186/s12263-020-00681-1.
89.Karpova, N. N. (2014) Role of BDNF epigenetics in
activity-dependent neuronal plasticity, Neuropharmacology, 76
Pt C, 709-718, doi: 10.1016/j.neuropharm.2013.04.002.
90.Irwin, R. E., Pentieva, K., Cassidy, T.,
Lees-Murdock, D. J., McLaughlin, M., Prasad, G., McNulty, H., and
Walsh, C. P. (2016) The interplay between DNA methylation, folate and
neurocognitive development, Epigenomics, 8, 863-879, doi:
10.2217/epi-2016-0003.
91.Martinowich, K., Hattori, D., Wu, H., Fouse, S.,
He, F., Hu, Y., Fan, G., and Sun, Y. E. (2003) DNA methylation-related
chromatin remodeling in activity-dependent BDNF gene regulation,
Science, 302, 890-893, doi: 10.1126/science.1090842.
92.Chen, W. G., Chang, Q., Lin, Y., Meissner, A.,
West, A. E., Griffith, E. C., Jaenisch, R., and Greenberg, M. E. (2003)
Derepression of BDNF transcription involves calcium-dependent
phosphorylation of MeCP2, Science, 302, 885-889, doi:
10.1126/science.1086446.
93.Feng, J., Fouse, S., and Fan, G. (2007)
Epigenetic regulation of neural gene expression and neuronal function,
Pediatr. Res., 61, 58R-63R, doi:
10.1203/pdr.0b013e3180457635.
94.Dhobale, M. V., Pisal, H. R., Mehendale, S. S.,
and Joshi, S. R. (2013) Differential expression of human placental
neurotrophic factors in preterm and term deliveries, Int. J. Dev.
Neurosci., 31, 719-723, doi:
10.1016/j.ijdevneu.2013.09.006.
95.Dhobale, M. (2017) Neurotrophic factors and
maternal nutrition during pregnancy, Vitam. Horm., 104,
343-366, doi: 10.1016/bs.vh.2016.10.011.
96.Geng, H., Chen, H., Wang, H., and Wang, L. (2021)
The histone modifications of neuronal plasticity, Neural.
Plast., 2021, 6690523, doi: 10.1155/2021/6690523.
97.Parrish, R. R., Buckingham, S. C., Mascia, K. L.,
Johnson, J. J., Matyjasik, M. M., Lockhart, R. M., and Lubin, F. D.
(2015) Methionine increases BDNF DNA methylation and improves memory in
epilepsy, Ann. Clin. Transl. Neurol., 2, 401-416, doi:
10.1002/acn3.183.
98.Xu, P., Pang, D., Zhou, J., Li, S., Chen, D., and
Yu, B. (2021) Behavioral changes and brain epigenetic alterations
induced by maternal deficiencies of B vitamins in a mouse model,
Psychopharmacology (Berl), 238, 1213-1222, doi:
10.1007/s00213-021-05766-2.
99.Yan, Z., Jiao, F., Yan, X., and Ou, H. (2017)
Maternal chronic folate supplementation ameliorates behavior disorders
induced by prenatal high-fat diet through methylation alteration of
BDNF and Grin2b in offspring hippocampus, Mol. Nutr. Food Res.,
61, 1700461, doi: 10.1002/mnfr.201700461.
100.Suganuma, T., and Workman, J. L. (2011) Signals
and combinatorial functions of histone modifications, Annu. Rev.
Biochem., 80, 473-499, doi:
10.1146/annurev-biochem-061809-175347.
101.Hyun, K., Jeon, J., Park, K., and Kim, J.
(2017) Writing, erasing and reading histone lysine methylations,
Exp. Mol. Med., 49, e324, doi: 10.1038/emm.2017.11.
102.Collins, B. E., Greer, C. B., Coleman, B. C.,
and Sweatt, J. D. (2019) Histone H3 lysine K4 methylation and its role
in learning and memory, Epigenetics Chromatin, 12, 7,
doi: 10.1186/s13072-018-0251-8.
103.Mentch, S. J., Mehrmohamadi, M., Huang, L.,
Liu, X., Gupta, D., Mattocks, D., Gomez Padilla, P., Ables, G., Bamman,
M. M., Thalacker-Mercer, A. E., Nichenametla, S. N., and Locasale, J.
W. (2015) Histone methylation dynamics and gene regulation occur
through the sensing of one-carbon metabolism, Cell Metab.,
22, 861-873, doi: 10.1016/j.cmet.2015.08.024.
104.Shen, W., Gao, C., Cueto, R., Liu, L., Fu, H.,
Shao, Y., Yang, W. Y., Fang, P., Choi, E. T., Wu, Q., Yang, X., and
Wang, H. (2020) Homocysteine-methionine cycle is a metabolic sensor
system controlling methylation-regulated pathological signaling,
Redox Biol., 28, 101322, doi:
10.1016/j.redox.2019.101322.
105.Nan, X., Ng, H. H., Johnson, C. A., Laherty, C.
D., Turner, B. M., Eisenman, R. N., and Bird, A. (1998) Transcriptional
repression by the methyl-CpG-binding protein MeCP2 involves a histone
deacetylase complex, Nature, 393, 386-389, doi:
10.1038/30764.
106.Barnes, C. E., English, D. M., and Cowley, S.
M. (2019) Acetylation & Co: an expanding repertoire of histone
acylations regulates chromatin and transcription, Essays
Biochem., 63, 97-107, doi: 10.1042/EBC20180061.
107.Rothbart, S. B., and Strahl, B. D. (2014)
Interpreting the language of histone and DNA modifications, Biochim.
Biophys. Acta, 1839, 627-643, doi:
10.1016/j.bbagrm.2014.03.001.
108.Gurda, D., Handschuh, L., Kotkowiak, W., and
Jakubowski, H. (2015) Homocysteine thiolactone and N-homocysteinylated
protein induce pro-atherogenic changes in gene expression in human
vascular endothelial cells, Amino Acids, 47, 1319-1339,
doi: 10.1007/s00726-015-1956-7.
109.Chaturvedi, P., Kalani, A., Givvimani, S.,
Kamat, P. K., Familtseva, A., and Tyagi, S. C. (2014) Differential
regulation of DNA methylation versus histone acetylation in
cardiomyocytes during HHcy in vitro and in vivo: an
epigenetic mechanism, Physiol. Genomics, 46, 245-255,
doi: 10.1152/physiolgenomics.00168.2013.
110.Tothova, B., Kovalska, M., Kalenska, D.,
Tomascova, A., and Lehotsky, J. (2018) Histone hyperacetylation as a
response to global brain ischemia associated with hyperhomocysteinemia
in rats, Int. J. Mol. Sci., 19, 3147, doi:
10.3390/ijms19103147.
111.Tremolizzo, L., Doueiri, M. S., Dong, E.,
Grayson, D. R., Davis, J., Pinna, G., Tueting, P., Rodriguez-Menendez,
V., Costa, E., and Guidotti, A. (2005) Valproate corrects the
schizophrenia-like epigenetic behavioral modifications induced by
methionine in mice, Biol. Psychiatry, 57, 500-509, doi:
10.1016/j.biopsych.2004.11.046.
112.Dong, E., Guidotti, A., Grayson, D. R., and
Costa, E. (2007) Histone hyperacetylation induces demethylation of
reelin and 67-kDa glutamic acid decarboxylase promoters, Proc. Natl.
Acad. Sci. USA, 104, 4676-4681, doi:
10.1073/pnas.0700529104.
113.Ma, Q., and Zhang, L. (2015) Epigenetic
programming of hypoxic-ischemic encephalopathy in response to fetal
hypoxia, Prog. Neurobiol., 124, 28-48, doi:
10.1016/j.pneurobio.2014.11.001.
114.Wellmann, S., Bettkober, M., Zelmer, A.,
Seeger, K., Faigle, M., Eltzschig, H. K., and Buhrer, C. (2008) Hypoxia
upregulates the histone demethylase JMJD1A via HIF-1, Biochem.
Biophys. Res. Commun., 372, 892-897, doi:
10.1016/j.bbrc.2008.05.150.
115.MacLennan, N. K., James, S. J., Melnyk, S.,
Piroozi, A., Jernigan, S., Hsu, J. L., Janke, S. M., Pham, T. D., and
Lane, R. H. (2004) Uteroplacental insufficiency alters DNA methylation,
one-carbon metabolism, and histone acetylation in IUGR rats,
Physiol. Genomics, 18, 43-50, doi:
10.1152/physiolgenomics.00042.2004.
116.Won, S. B., Han, A., and Kwon, Y. H. (2017)
Maternal consumption of low-isoflavone soy protein isolate alters
hepatic gene expression and liver development in rat offspring, J.
Nutr. Biochem., 42, 51-61, doi:
10.1016/j.jnutbio.2016.12.013.
117.Durbagula, S., Korlimarla, A., Ravikumar, G.,
Valiya Parambath, S., Kaku, S. M., and Visweswariah, A. M. (2022)
Prenatal epigenetic factors are predisposing for neurodevelopmental
disorders – considering placenta as a model, Birth Defects
Res., 114, 1324-1342, doi: 10.1002/bdr2.2119.
118.Rosenfeld, C. S. (2021) The
placenta-brain-axis, J. Neurosci. Res., 99, 271-283, doi:
10.1002/jnr.24603.
119.Nelissen, E.C., van Montfoort, A.P., Dumoulin,
J.C., and Evers, J.L. (2011) Epigenetics and the placenta, Hum.
Reprod. Update, 17, 397-417, doi: 10.1093/humupd/dmq052.
120.Deshpande, S.S., and Balasinor, N.H. (2018)
Placental Defects: An Epigenetic Perspective, Reprod. Sci.,
25, 1143-1160, doi: 10.1177/1933719118766265.
121.D’Souza, S. W., and Glazier, J. D. (2022)
Homocysteine metabolism in pregnancy and developmental impacts,
Front. Cell Dev. Biol., 10, 802285, doi:
10.3389/fcell.2022.802285.
122.Geng, Y., Gao, R., Chen, X., Liu, X., Liao, X.,
Li, Y., Liu, S., Ding, Y., Wang, Y., and He, J. (2015) Folate
deficiency impairs decidualization and alters methylation patterns of
the genome in mice, Mol. Hum. Reprod., 21, 844-856, doi:
10.1093/molehr/gav045.
123.Kim, J. M., Hong, K., Lee, J. H., Lee, S., and
Chang, N. (2009) Effect of folate deficiency on placental DNA
methylation in hyperhomocysteinemic rats, J. Nutr. Biochem.,
20, 172-176, doi: 10.1016/j.jnutbio.2008.01.010.
124.Park, B. H., Kim, Y. J., Park, J. S., Lee, H.
Y., Ha, E. H., Min, J. W., and Park, H. S. (2005) Folate and
homocysteine levels during pregnancy affect DNA methylation in human
placenta, J. Prev. Med. Public Health, 38, 437-442.
125.Kulkarni, A., Dangat, K., Kale, A., Sable, P.,
Chavan-Gautam, P., and Joshi, S. (2011) Effects of altered maternal
folic acid, vitamin B12 and docosahexaenoic acid on
placental global DNA methylation patterns in Wistar rats, PLoS
One, 6, e17706, doi: 10.1371/journal.pone.0017706.
126.Li, B., Chang, S., Liu, C., Zhang, M., Zhang,
L., Liang, L., Li, R., Wang, X., Qin, C., Zhang, T., Niu, B., and Wang,
L. (2019) Low maternal dietary folate alters retrotranspose by
methylation regulation in intrauterine growth retardation (IUGR)
fetuses in a mouse model, Med. Sci. Monit., 25,
3354-3365, doi: 10.12659/MSM.914292.
127.Dai, C., Fei, Y., Li, J., Shi, Y., and Yang, X.
(2021) A novel review of homocysteine and pregnancy complications,
Biomed. Res. Int., 2021, 6652231, doi:
10.1155/2021/6652231.
128.Nomura, Y., Lambertini, L., Rialdi, A., Lee,
M., Mystal, E. Y., Grabie, M., Manaster, I., Huynh, N., Finik, J.,
Davey, M., Davey, K., Ly, J., Stone, J., Loudon, H., Eglinton, G.,
Hurd, Y., Newcorn, J. H., and Chen, J. (2014) Global methylation in the
placenta and umbilical cord blood from pregnancies with maternal
gestational diabetes, preeclampsia, and obesity, Reprod. Sci.,
21, 131-137, doi: 10.1177/1933719113492206.
129.Lecuyer, M., Laquerriere, A., Bekri, S.,
Lesueur, C., Ramdani, Y., Jegou, S., Uguen, A., Marcorelles, P.,
Marret, S., and Gonzalez, B. J. (2017) PLGF, a placental marker of
fetal brain defects after in utero alcohol exposure, Acta
Neuropathol. Commun., 5, 44, doi:
10.1186/s40478-017-0444-6.
130.Rahat, B., Hamid, A., Bagga, R., and Kaur, J.
(2022) Folic acid levels during pregnancy regulate trophoblast invasive
behavior and the possible development of preeclampsia, Front.
Nutr., 9, 847136, doi: 10.3389/fnut.2022.847136.
131.Meister, S., Hahn, L., Beyer, S., Paul, C.,
Mitter, S., Kuhn, C., von Schonfeldt, V., Corradini, S., Sudan, K.,
Schulz, C., Kolben, T. M., Mahner, S., Jeschke, U., and Kolben, T.
(2021) Regulation of epigenetic modifications in the placenta during
preeclampsia: PPARgamma influences H3K4me3 and H3K9ac in extravillous
trophoblast cells, Int. J. Mol. Sci., 22, 12469, doi:
10.3390/ijms222212469.
132.Sheng, W., Gu, Y., Chu, X., Morgan, J. A.,
Cooper, D. B., Lewis, D. F., McCathran, C. E., and Wang, Y. (2021)
Upregulation of histone H3K9 methylation in fetal endothelial cells
from preeclamptic pregnancies, J. Cell. Physiol., 236,
1866-1874, doi: 10.1002/jcp.29970.
133.Morales-Prieto, D. M., Chaiwangyen, W.,
Ospina-Prieto, S., Schneider, U., Herrmann, J., Gruhn, B., and Markert,
U. R. (2012) MicroRNA expression profiles of trophoblastic cells,
Placenta, 33, 725-734, doi:
10.1016/j.placenta.2012.05.009.
134.Xie, L., Mouillet, J. F., Chu, T., Parks, W.
T., Sadovsky, E., Knofler, M., and Sadovsky, Y. (2014) C19MC microRNAs
regulate the migration of human trophoblasts, Endocrinology,
155, 4975-4985, doi: 10.1210/en.2014-1501.
135.Liang, Y., Ridzon, D., Wong, L., and Chen, C.
(2007) Characterization of microRNA expression profiles in normal human
tissues, BMC Genomics, 8, 166, doi:
10.1186/1471-2164-8-166.
136.Gunel, T., Kamali, N., Hosseini, M. K.,
Gumusoglu, E., Benian, A., and Aydinli, K. (2020) Regulatory effect of
miR-195 in the placental dysfunction of preeclampsia, J. Matern.
Fetal Neonatal Med., 33, 901-908, doi:
10.1080/14767058.2018.1508439.
137.Li, J., Du, J., Wang, Z., Wang, C., Bai, J.,
and Zhang, S. (2018) Expression of miR-376 in blood of pregnant women
with preeclampsia and its effect on 25-hydroxyvitamin D, Exp. Ther.
Med., 16, 1701-1706, doi: 10.3892/etm.2018.6394.
138.Lv, Y., Lu, C., Ji, X., Miao, Z., Long, W.,
Ding, H., and Lv, M. (2019) Roles of microRNAs in preeclampsia, J.
Cell. Physiol., 234, 1052-1061, doi: 10.1002/jcp.27291.
139.Cai, M., Kolluru, G. K., and Ahmed, A. (2017)
Small molecule, big prospects: microRNA in pregnancy and its
complications, J. Pregnancy, 2017, 6972732, doi:
10.1155/2017/6972732.
140.Chen, D. B., and Wang, W. (2013) Human
placental microRNAs and preeclampsia, Biol. Reprod., 88,
130, doi: 10.1095/biolreprod.113.107805.
141.Fu, G., Brkic, J., Hayder, H., and Peng, C.
(2013) MicroRNAs in human placental development and pregnancy
complications, Int. J. Mol. Sci., 14, 5519-5544, doi:
10.3390/ijms14035519.
142.Escudero, C. A., Herlitz, K., Troncoso, F.,
Acurio, J., Aguayo, C., Roberts, J. M., Truong, G., Duncombe, G., Rice,
G., and Salomon, C. (2016) Role of extracellular vesicles and microRNAs
on dysfunctional angiogenesis during preeclamptic pregnancies,
Front. Physiol., 7, 98, doi:
10.3389/fphys.2016.00098.
143.Rudov, A., Balduini, W., Carloni, S., Perrone,
S., Buonocore, G., and Albertini, M. C. (2014) Involvement of miRNAs in
placental alterations mediated by oxidative stress, Oxid. Med. Cell.
Longev., 2014, 103068, doi: 10.1155/2014/103068.
144.Sonkar, R., Kay, M. K., and Choudhury, M.
(2019) PFOS modulates interactive epigenetic regulation in
first-trimester human trophoblast cell line HTR-8/SVneo, Chem. Res.
Toxicol., 32, 2016-2027, doi:
10.1021/acs.chemrestox.9b00198.
145.Gold, A. R., and Glanzman, D. L. (2021) The
central importance of nuclear mechanisms in the storage of memory,
Biochem. Biophys. Res. Commun., 564, 103-113, doi:
10.1016/j.bbrc.2021.04.125.
146.Jobe, E. M., and Zhao, X. (2017) DNA
methylation and adult neurogenesis, Brain Plast., 3,
5-26, doi: 10.3233/BPL-160034.
147.Graff, J., and Mansuy, I. M. (2008) Epigenetic
codes in cognition and behaviour, Behav. Brain Res., 192,
70-87, doi: 10.1016/j.bbr.2008.01.021.
148.Wood, M. A., Hawk, J. D., and Abel, T. (2006)
Combinatorial chromatin modifications and memory storage: a code for
memory? Learn Mem., 13, 241-244, doi:
10.1101/lm.278206.
149.Hwang, J. Y., Aromolaran, K. A., and Zukin, R.
S. (2017) The emerging field of epigenetics in neurodegeneration and
neuroprotection, Nat. Rev. Neurosci., 18, 347-361, doi:
10.1038/nrn.2017.46.
150.Duman, R. S., and Aghajanian, G. K. (2012)
Synaptic dysfunction in depression: potential therapeutic targets,
Science, 338, 68-72, doi: 10.1126/science.1222939.
151.Uchida, S., Yamagata, H., Seki, T., and
Watanabe, Y. (2018) Epigenetic mechanisms of major depression:
targeting neuronal plasticity, Psychiatry Clin. Neurosci.,
72, 212-227, doi: 10.1111/pcn.12621.
152.Kubota, T., Miyake, K., Hariya, N., and
Mochizuki, K. (2015) Understanding the epigenetics of
neurodevelopmental disorders and DOHaD, J. Dev. Orig. Health
Dis., 6, 96-104, doi: 10.1017/S2040174415000057.
153.Matrisciano, F., Tueting, P., Dalal, I.,
Kadriu, B., Grayson, D. R., Davis, J. M., Nicoletti, F., and Guidotti,
A. (2013) Epigenetic modifications of GABAergic interneurons are
associated with the schizophrenia-like phenotype induced by prenatal
stress in mice, Neuropharmacology, 68, 184-194, doi:
10.1016/j.neuropharm.2012.04.013.
154.Weaver, I. C., Cervoni, N., Champagne, F. A.,
D’Alessio, A. C., Sharma, S., Seckl, J. R., Dymov, S., Szyf, M.,
and Meaney, M. J. (2004) Epigenetic programming by maternal behavior,
Nat. Neurosci., 7, 847-854, doi: 10.1038/nn1276.
155.Kubota, T. (2018) Preemptive epigenetic
medicine based on fetal programming, Adv. Exp. Med. Biol.,
1012, 85-95, doi: 10.1007/978-981-10-5526-3_9.
156.Arutyunyan, A. V., Zaloznyaya, I. V.,
Kerkeshko, G. O., Milyutina, Y. P., and Korenevskii, A. V. (2017)
Prenatal hyperhomocysteinemia impairs hypothalamic regulation of
reproductive cycles in rat progeny, Bull. Exp. Biol. Med.,
162, 738-740, doi: 10.1007/S10517-017-3701-6.
157.Wang, X., Li, W., Li, Z., Ma, Y., Yan, J.,
Wilson, J. X., and Huang, G. (2019) Maternal folic acid supplementation
during pregnancy promotes neurogenesis and synaptogenesis in neonatal
rat offspring, Cereb. Cortex, 29, 3390-3397, doi:
10.1093/cercor/bhy207.
158.Loureiro, S. O., Heimfarth, L., Pelaez Pde, L.,
Vanzin, C. S., Viana, L., Wyse, A. T., and Pessoa-Pureur, R. (2008)
Homocysteine activates calcium-mediated cell signaling mechanisms
targeting the cytoskeleton in rat hippocampus, Int. J. Dev.
Neurosci., 26, 447-455, doi:
10.1016/j.ijdevneu.2008.03.001.
159.Lockhart, B., Jones, C., Cuisinier, C.,
Villain, N., Peyroulan, D., and Lestage, P. (2000) Inhibition of
L-homocysteic acid and buthionine sulphoximine-mediated neurotoxicity
in rat embryonic neuronal cultures with alpha-lipoic acid enantiomers,
Brain Res., 855, 292-297, doi:
10.1016/s0006-8993(99)02372-0.
160.Alzoubi, K.H., Aburashed, Z.O., and Mayyas, F.
(2020) Edaravone protects from memory impairment induced by chronic
L-methionine administration, Naunyn Schmiedebergs Arch.
Pharmacol., 393, 1221-1228, doi:
10.1007/s00210-020-01827-z.
161.Milyutina, Yu. P., Arutjunyan, A. V.,
Pustygina, A. V., Shcherbitskaya, A. D., Zaloznyaya, I. V., and Zorina,
I. I. (2014) Content of catecholamines in the adrenal gland of rat pups
subjected to prenatal hyperhomocysteinemia, Ross. Physiol. Zhurn.
im. I. M. Sechenova, 100, 360-369.
162.Algaidi, S. A., Christie, L. A., Jenkinson, A.
M., Whalley, L., Riedel, G., and Platt, B. (2006) Long-term
homocysteine exposure induces alterations in spatial learning,
hippocampal signalling and synaptic plasticity, Exp. Neurol.,
197, 8-21, doi: 10.1016/j.expneurol.2005.07.003.
163.Christie, L. A., Riedel, G., Algaidi, S. A.,
Whalley, L. J., and Platt, B. (2005) Enhanced hippocampal long-term
potentiation in rats after chronic exposure to homocysteine,
Neurosci. Lett., 373, 119-124, doi:
10.1016/j.neulet.2004.09.072.
164.Viggiano, A., Viggiano, E., Monda, M.,
Ingrosso, D., Perna, A. F., and De Luca, B. (2012) Methionine-enriched
diet decreases hippocampal antioxidant defences and impairs spontaneous
behaviour and long-term potentiation in rats, Brain Res.,
1471, 66-74, doi: 10.1016/j.brainres.2012.06.048.
165.Christie, L. A., Riedel, G., and Platt, B.
(2009) Bi-directional alterations of LTP after acute homocysteine
exposure, Behav. Brain Res., 205, 559-563, doi:
10.1016/j.bbr.2009.07.010.
166.Chai, G. S., Jiang, X., Ni, Z. F., Ma, Z. W.,
Xie, A. J., Cheng, X. S., Wang, Q., Wang, J. Z., and Liu, G. P. (2013)
Betaine attenuates Alzheimer-like pathological changes and memory
deficits induced by homocysteine, J. Neurochem., 124,
388-396, doi: 10.1111/jnc.12094.
167.Zeng, P., Shi, Y., Wang, X. M., Lin, L., Du, Y.
J., Tang, N., Wang, Q., Fang, Y. Y., Wang, J. Z., Zhou, X. W., Lu, Y.,
and Tian, Q. (2019) Emodin rescued hyperhomocysteinemia-induced
dementia and Alzheimer’s disease-like features in rats, Int.
J. Neuropsychopharmacol., 22, 57-70, doi:
10.1093/ijnp/pyy090.
168.Mahaman, Y. A. R., Huang, F., Wu, M., Wang, Y.,
Wei, Z., Bao, J., Salissou, M. T. M., Ke, D., Wang, Q., Liu, R., Wang,
J. Z., Zhang, B., Chen, D., and Wang, X. (2018) Moringa Oleifera
alleviates homocysteine-induced Alzheimer’s disease-like
pathology and cognitive impairments, J. Alzheimers Dis.,
63, 1141-1159, doi: 10.3233/JAD-180091.
169.Salissou, M. T. M., Mahaman, Y. A. R., Zhu, F.,
Huang, F., Wang, Y., Xu, Z., Ke, D., Wang, Q., Liu, R., Wang, J. Z.,
Zhang, B., and Wang, X. (2018) Methanolic extract of Tamarix Gallica
attenuates hyperhomocysteinemia induced AD-like pathology and cognitive
impairments in rats, Aging (Albany NY), 10, 3229-3248,
doi: 10.18632/aging.101627.
170.Kamat, P. K., Kyles, P., Kalani, A., and Tyagi,
N. (2016) Hydrogen sulfide ameliorates homocysteine-induced
Alzheimer’s disease-like pathology, blood-brain barrier
disruption, and synaptic disorder, Mol. Neurobiol., 53,
2451-2467, doi: 10.1007/s12035-015-9212-4.
171.Li, J. G., Barrero, C., Merali, S., and
Pratico, D. (2017) Genetic absence of ALOX5 protects from
homocysteine-induced memory impairment, tau phosphorylation and
synaptic pathology, Hum. Mol. Genet., 26, 1855-1862, doi:
10.1093/hmg/ddx088.
172.Li, J. G., Barrero, C., Merali, S., and
Pratico, D. (2017) Five lipoxygenase hypomethylation mediates the
homocysteine effect on Alzheimer’s phenotype, Sci. Rep.,
7, 46002, doi: 10.1038/srep46002.
173.Akchiche, N., Bossenmeyer-Pourie, C., Kerek,
R., Martin, N., Pourie, G., Koziel, V., Helle, D., Alberto, J. M.,
Ortiou, S., Camadro, J. M., Leger, T., Gueant, J. L., and Daval, J. L.
(2012) Homocysteinylation of neuronal proteins contributes to folate
deficiency-associated alterations of differentiation, vesicular
transport, and plasticity in hippocampal neuronal cells, FASEB
J., 26, 3980-3992, doi: 10.1096/fj.12-205757.
174.Sibarov, D. A., Giniatullin, R., and Antonov,
S. M. (2018) High sensitivity of cerebellar neurons to homocysteine is
determined by expression of GluN2C and GluN2D subunits of NMDA
receptors, Biochem. Biophys. Res. Commun., 506, 648-652,
doi: 10.1016/j.bbrc.2018.10.140.
175.Choe, Y. M., Sohn, B. K., Choi, H. J., Byun, M.
S., Seo, E. H., Han, J. Y., Kim, Y. K., Yoon, E. J., Lee, J. M., Park,
J., Woo, J. I., and Lee, D. Y. (2014) Association of homocysteine with
hippocampal volume independent of cerebral amyloid and vascular burden,
Neurobiol. Aging, 35, 1519-1525, doi:
10.1016/j.neurobiolaging.2014.01.013.
176.Duan, W., Ladenheim, B., Cutler, R. G., Kruman,
I. I., Cadet, J. L., and Mattson, M. P. (2002) Dietary folate
deficiency and elevated homocysteine levels endanger dopaminergic
neurons in models of Parkinson’s disease, J. Neurochem.,
80, 101-110, doi: 10.1046/j.0022-3042.2001.00676.x.
177.Alachkar, A., Wang, L., Yoshimura, R., Hamzeh,
A. R., Wang, Z., Sanathara, N., Lee, S. M., Xu, X., Abbott, G. W., and
Civelli, O. (2018) Prenatal one-carbon metabolism dysregulation
programs schizophrenia-like deficits, Mol. Psychiatry,
23, 282-294, doi: 10.1038/mp.2017.164.
178.Zhuo, J. M., Wang, H., and Pratico, D. (2011)
Is hyperhomocysteinemia an Alzheimer’s disease (AD) risk factor,
an AD marker, or neither? Trends Pharmacol. Sci., 32,
562-571, doi: 10.1016/j.tips.2011.05.003.
179.Velazquez, R., Ferreira, E., Winslow, W., Dave,
N., Piras, I. S., Naymik, M., Huentelman, M. J., Tran, A., Caccamo, A.,
and Oddo, S. (2020) Maternal choline supplementation ameliorates
Alzheimer's disease pathology by reducing brain homocysteine levels
across multiple generations, Mol. Psychiatry, 25,
2620-2629, doi: 10.1038/s41380-018-0322-z.
180.Da Silva, V. C., Fernandes, L., Haseyama, E.
J., Agamme, A. L., Guerra Shinohara, E. M., Muniz, M. T., and
D’Almeida, V. (2014) Effect of vitamin B deprivation during
pregnancy and lactation on homocysteine metabolism and related
metabolites in brain and plasma of mice offspring, PLoS One,
9, e92683, doi: 10.1371/journal.pone.0092683.
181.Gulyaeva, N. V. (2017) Molecular mechanisms of
neuroplasticity: an expanding universe, Biochemistry (Moscow),
82, 237-242, doi: 10.1134/S0006297917030014.
182.Balaban, P. M. and Gulyaeva, N. V. (2006)
Common molecular mechanisms of neuroplasticity and neuropathology: an
integrative approach, Ross. Physiol. Zhurn. im. I. M. Sechenova,
92, 145-151.
183.Bacon, E. R., and Brinton, R. D. (2021)
Epigenetics of the developing and aging brain: Mechanisms that regulate
onset and outcomes of brain reorganization, Neurosci. Biobehav.
Rev., 125, 503-516, doi:
10.1016/j.neubiorev.2021.02.040.
184.Liu, X. S., and Jaenisch, R. (2019) Editing the
epigenome to tackle brain disorders, Trends Neurosci.,
42, 861-870, doi: 10.1016/j.tins.2019.10.003.
185.Sheikhi, S., and Saboory, E. (2015)
Neuroplasticity changes of rat brain by musical stimuli during fetal
period, Cell J., 16, 448-455, doi:
10.22074/cellj.2015.490.
186.Gao, J., Cahill, C. M., Huang, X., Roffman, J.
L., Lamon-Fava, S., Fava, M., Mischoulon, D., and Rogers, J. T. (2018)
S-Adenosyl methionine and transmethylation pathways in neuropsychiatric
diseases throughout life, Neurotherapeutics, 15, 156-175,
doi: 10.1007/s13311-017-0593-0.
187.Taylor Levine, M., Gao, J., Satyanarayanan, S.
K., Berman, S., Rogers, J. T., and Mischoulon, D. (2020)
S-adenosyl-l-methionine (SAMe), cannabidiol (CBD), and kratom in
psychiatric disorders: clinical and mechanistic considerations,
Brain Behav. Immun., 85, 152-161, doi:
10.1016/j.bbi.2019.07.013.
188.Remington, R., Bechtel, C., Larsen, D., Samar,
A., Doshanjh, L., Fishman, P., Luo, Y., Smyers, K., Page, R., Morrell,
C., and Shea, T. B. (2015) A phase II randomized clinical trial of a
nutritional formulation for cognition and mood in Alzheimer’s
disease, J. Alzheimers Dis., 45, 395-405, doi:
10.3233/JAD-142499.
189.Gupta, R., Ambasta, R. K., and Kumar, P. (2021)
Histone deacetylase in neuropathology, Adv. Clin. Chem.,
104, 151-231, doi: 10.1016/bs.acc.2020.09.004.
190.Fontana, A., Cursaro, I., Carullo, G., Gemma,
S., Butini, S., and Campiani, G. (2022) A therapeutic perspective of
HDAC8 in different diseases: an overview of selective inhibitors,
Int. J. Mol. Sci., 23, 10014, doi:
10.3390/ijms231710014.
191.Ricci, R., and Colasante, G. (2021)
CRISPR/dCas9 as a therapeutic approach for neurodevelopmental
disorders: innovations and limitations compared to traditional
strategies, Dev. Neurosci., 43, 253-261, doi:
10.1159/000515845.
192.Peter, C.J., Saito, A., Hasegawa, Y., Tanaka,
Y., Nagpal, M., Perez, G., Alway, E., Espeso-Gil, S., Fayyad, T.,
Ratner, C., Dincer, A., Gupta, A., Devi, L., Pappas, J. G., Lalonde,
F.M., Butman, J.A., Han, J.C., Akbarian, S., and Kamiya, A. (2019) In
vivo epigenetic editing of Sema6a promoter reverses transcallosal
dysconnectivity caused by C11orf46/Arl14ep risk gene, Nat.
Commun., 10, 4112, doi: 10.1038/s41467-019-12013-y.
193.Liu, X.S., Wu, H., Krzisch, M., Wu, X., Graef,
J., Muffat, J., Hnisz, D., Li, C.H., Yuan, B., Xu, C., Li, Y.,
Vershkov, D., Cacace, A., Young, R.A., and Jaenisch, R. (2018) Rescue
of fragile X syndrome neurons by DNA methylation editing of the FMR1
gene, Cell, 172, 979-992.e6, doi:
10.1016/j.cell.2018.01.012.
194.Monayo, S. M., and Liu, X. (2022) The
prospective application of melatonin in treating epigenetic
dysfunctional diseases, Front. Pharmacol., 13, 867500,
doi: 10.3389/fphar.2022.867500.
195.Alghamdi, B. S. (2018) The neuroprotective role
of melatonin in neurological disorders, J. Neurosci. Res.,
96, 1136-1149, doi: 10.1002/jnr.24220.
196.Leung, J. W., Cheung, K. K., Ngai, S. P.,
Tsang, H. W., and Lau, B. W. (2020) Protective effects of melatonin on
neurogenesis impairment in neurological disorders and its relevant
molecular mechanisms, Int. J. Mol. Sci., 21, 5645, doi:
10.3390/ijms21165645.
197.Figueiro-Silva, J., Antequera, D., Pascual, C.,
de la Fuente Revenga, M., Volt, H., Acuna-Castroviejo, D.,
Rodriguez-Franco, M. I., and Carro, E. (2018) The melatonin analog
IQM316 may induce adult hippocampal neurogenesis and preserve
recognition memories in mice, Cell Transplant., 27,
423-437, doi: 10.1177/0963689717721217.
198.Ghareghani, M., Sadeghi, H., Zibara, K.,
Danaei, N., Azari, H., and Ghanbari, A. (2017) Melatonin increases
oligodendrocyte differentiation in cultured neural stem cells, Cell.
Mol. Neurobiol., 37, 1319-1324, doi:
10.1007/s10571-016-0450-4.
199.Li, X., Chen, X., Zhou, W., Ji, S., Li, X., Li,
G., Liu, G., Wang, F., and Hao, A. (2017) Effect of melatonin on
neuronal differentiation requires CBP/p300-mediated acetylation of
histone H3 lysine 14, Neuroscience, 364, 45-59, doi:
10.1016/j.neuroscience.2017.07.064.
200.Liu, Y., Zhang, Z., Lv, Q., Chen, X., Deng, W.,
Shi, K., and Pan, L. (2016) Effects and mechanisms of melatonin on the
proliferation and neural differentiation of PC12 cells, Biochem.
Biophys. Res. Commun., 478, 540-545, doi:
10.1016/j.bbrc.2016.07.093.
201.Kong, X., Li, X., Cai, Z., Yang, N., Liu, Y.,
Shu, J., Pan, L., and Zuo, P. (2008) Melatonin regulates the viability
and differentiation of rat midbrain neural stem cells, Cell. Mol.
Neurobiol., 28, 569-579, doi: 10.1007/s10571-007-9212-7.
202.Paul, R., and Borah, A. (2015) The potential
physiological crosstalk and interrelationship between two sovereign
endogenous amines, melatonin and homocysteine, Life Sci.,
139, 97-107, doi: 10.1016/j.lfs.2015.07.031.
203.Karolczak, K., and Watala, C. (2021) Melatonin
as a reducer of neuro- and vasculotoxic oxidative stress induced by
homocysteine, Antioxidants (Basel), 10, 1178, doi:
10.3390/antiox10081178.
204.Liu, J., Mesfin, F. M., Hunter, C. E., Olson,
K. R., Shelley, W. C., Brokaw, J. P., Manohar, K., and Markel, T. A.
(2022) Recent development of the molecular and cellular mechanisms of
hydrogen sulfide gasotransmitter, Antioxidants (Basel),
11, 1788, doi: 10.3390/antiox11091788.