REVIEW: Photoactive Pigment-Enzyme Complexes of Chlorophyll Precursor in Plant Leaves
O. B. Belyaeva* and F. F. Litvin
Faculty of Biology, Lomonosov Moscow State University, 119991 Moscow, Russia; E-mail: olgabelyaeva@mail.ru* To whom correspondence should be addressed.
Received November 22, 2006
This review summarizes contemporary data on structure and function of photoactive pigment-enzyme complexes of the chlorophyll precursor that undergoes photochemical transformation to chlorophyllide. The properties and functions of the complex and its principal components are considered including the pigment (protochlorophyllide), the hydrogen donor (NADPH), and the photoenzyme protochlorophyllide oxidoreductase (POR) that catalyzes the photochemical production of chlorophyllide. Chemical variants of the chlorophyll precursor are described (protochlorophyllide, protochlorophyll, and their mono- and divinyl forms). The nature and photochemical activity of spectrally distinct native protochlorophyllide forms are discussed. Data are presented on structural organization of the photoenzyme POR, its substrate specificity, localization in etioplasts, and heterogeneity. The significance of different POR forms (PORA, PORB, and PORC) in adaptation of chlorophyll biosynthesis to various illumination conditions is considered. Attention is paid to structural and functional interactions of three main constituents of the photoactive complex and to possible existence of additional components associated with the pigment-enzyme complex. Historical aspects of the problem and the prospects of further investigations are outlined.
KEY WORDS: chlorophyll biosynthesis, photoreduction of protochlorophyllide, protochlorophyllide oxidoreductase, pigment-protein complexesDOI: 10.1134/S0006297907130044
Abbreviations: Pchl) protochlorophyll; Pchlide) protochlorophyllide; PLB) prolamellar bodies; POR) protochlorophyllide oxidoreductase; PS1, PS2) photosystems 1 and 2, respectively.
Chlorophyll biosynthesis is fundamental for the formation of the
photosynthetic apparatus that ensures photosynthesis and, consequently,
the existence of the biosphere. Biosynthesis of chlorophyll from its
precursor, protochlorophyll(ide), can proceed through two pathways. A
more primitive pathway is light-independent (dark) transformation of
protochlorophyll(ide) to chlorophyll(ide). The dark chlorophyll
synthesis from the precursor was observed in cyanobacteria, green
algae, mosses, ferns, and gymnospermous plants. The other pathway
includes a finely regulated terminal stage of chlorophyll biosynthesis
from the precursor and is accomplished during the light-dependent
reaction (this pathway operates in all higher plants, some lower
plants, and gymnosperms). The light-dependent terminal stage of
chlorophyll biosynthesis has long been a matter of keen interest. For
example, the instant appearance of green color during illumination of
dark-grown (etiolated) seedlings can be seen with the naked eye.
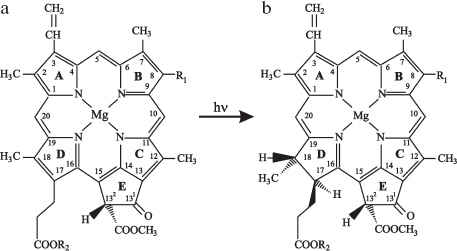
The immediate precursor of chlorophyll is protochlorophyll, specifically, its phytol-free form, protochlorophyllide (Pchlide). The chlorophyll(ide) and protochlorophyll(ide) structures (Fig. 1) were established by Fischer in a series of studies based on analytical and synthetic approaches [1-3].
Chlorophyll and its precursor are magnesium-containing porphyrins. These molecules are closed tetrapyrroles with a system of conjugated double bonds (pyrrole rings A, B, C, and D). Four pyrrole rings are linked with methine bridges; the periphery of the large ring carries eight substituent groups. The additional homocyclic ring E contains carbonyl oxygen and methanol-esterified carboxylic group. The protochlorophyll(ide) molecule differs from chlorophyll(ide) by the absence of two hydrogen atoms at positions 17-18 in the fourth pyrrole ring (ring D) of the macrocycle (Fig. 1) [1-3]. Thus, the terminal stage of chlorophyll biosynthesis is based on the photochemical hydrogenation of a semi-isolated double bond C17=C18 in the precursor molecule. The reduction of this bond in the porphyrin macrocycle alters the molecule symmetry and the absorption spectrum causing a sharp increase of the long-wavelength band in the red spectral region. The new property of the chromophore (chlorine type) not only increases the capacity of the molecule for light absorption in a broad spectral region, but also determines its functional activity: trapping light energy, excitation energy transfer, and charge separation in photosynthetic reaction centers.Fig. 1. Structures of (a) protochlorophyll(ide) and (b) chlorophyll(ide) molecules. R1 is CH2-CH3 for the monovinyl pigment form and CH=CH2 for the divinyl form. R2 is C20H39 for protochlorophyll and chlorophyll and a hydrogen atom for protochlorophyllide and chlorophyllide.
The inclusion of a light-dependent stage providing for regulated rates of biosynthesis can be considered as an important evolutionary advance of all higher plants. The light stage allows regulation of not only chlorophyll biosynthesis but also the whole plant development. Seed germination and shoot emergence are accompanied by rapid (a few hours) formation of the complete photosynthetic apparatus that makes the plant capable of self-sufficient autotrophic life.
In early studies, the terminal stage of chlorophyll biosynthesis was considered in a purely chemical aspect, as an elementary photochemical reaction that accomplishes the construction of the pigment molecule. However, the progress of these investigations led to recognition that the reaction participants, similarly to those in photosynthesis, are sophisticated pigment-protein complexes rather than isolated chromophores. The problem of chlorophyll biosynthesis is integrated into a more general problem of biogenesis of functionally active structures of the photosynthetic apparatus. In this connection, a question arises on specific pathways involved in biogenesis of pigment-protein complexes of the antenna and reaction centers of two photochemical systems of photosynthesis.
The conversion of protochlorophyllide to chlorophyllide has been realized comparatively recently in artificially assembled complexes. However, the phototransformation of pigment-protein complexes in plant leaves turned out to be much more complicated than in the model systems.
According to schemes proposed by various authors, chlorophyll biosynthesis in the live cell is a branched multistage process that includes several pathways for transformation of chlorophyll precursor complexes to pigment-protein complexes of chlorophyll. The complexity of chlorophyll photobiosynthesis in the live cell is largely determined by the structure, condition, and heterogeneity of chlorophyll precursor pigment-protein complexes produced prior to the light-dependent stage. This review considers the structure and function of photoactive protochlorophyll(ide) complexes and their role in chlorophyll formation.
HISTORICAL VIEW ON STUDIES OF PROTOCHLOROPHYLL PIGMENT-PROTEIN
COMPLEX
The discovery of chlorophyll precursor and early studies of its conversion to chlorophyll are largely due to Russian scientists K. A. Timiryazev, N. A. Monteverde, and V. N. Lyubimenko (a short historical survey of studies by Russian investigators is presented in [4]).
A characteristic feature of chlorophyll formation is its exclusive occurrence in integral biological systems such as leaves and leaf homogenates. The photochemical conversion of protochlorophyll(ide) to chlorophyll(ide) in simple systems (solutions) has not been realized so far. Already the earliest studies established that chlorophyll biosynthesis occurs in specialized native complexes of the pigment and some carrier providing for physiological activity and spectral properties in vivo. Lyubimenko put forward an idea that specificity of protochlorophyll and chlorophyll in plants is determined by the existence of some pigment-protein compound [5, 6]. Physiologically active native complexes were termed holochromes (from Greek words “holos”, whole and “chroma”, color) [7, 8]. Krasnovsky and Kosobutskaya were the first to extract these active complexes of protochlorophyllide in aqueous medium [9, 10]. In thus obtained “colloid solutions” of the substance from etiolated leaves, protochlorophyll had a characteristic absorption band at 637 nm, whereas the absorption spectra of whole etiolated leaves contained also the long-wavelength maximum at 650 nm. Protochlorophyll in colloid solutions retained its physiological activity and transformed to chlorophyll under the action of light.
Smith [8, 11] succeeded in isolating a protochlorophyll-protein complex using glycerol. The position of main absorption bands in isolated “protochlorophyll-holochrome” particles depended on plant species and temperature conditions during the isolation procedure. Smith and Benitez [12] investigated temperature dependence of protochlorophyll to chlorophyll conversion and found that the temperature coefficient for inactivation of this reaction was the same as the temperature coefficient for denaturation of some proteins. In later studies, protochlorophyll-protein complexes were obtained by means of differential centrifugation [13-17]. The absorption spectra of these complexes were similar to the spectrum of whole etiolated leaves (maxima at 637 and 650 nm); however, the short-wavelength peak was more intense.
Later works established that the chlorophyll precursor in plant leaves is a phytol-free pigment form, protochlorophyllide, and that the photochemical reaction represents the transformation of protochlorophyllide to chlorophyllide [18, 19].
PHOTOENZYME NADPH-PROTOCHLOROPHYLLIDE-OXIDOREDUCTASE (POR)
Experiments performed in the 1970s in the laboratory of Prof. Griffiths at the University of Bristol clarified the nature of the hydrogen donor in the reaction of protochlorophyllide photoreduction and the composition of the active pigment complex. Griffiths found that the addition of NADPH to etioplast membranes from maize and barley seedlings resulted in transformation of inactive protochlorophyllide (absorption maximum at 630 nm) to its active forms with absorption maxima around 640 and 652 nm [20, 21]. He considered possible formation of the active ternary complex NADPH-protein-Pchlide, in which NADPH serves as a reductant for the conversion of Pchlide to chlorophyllide [20]. By applying exogenous protochlorophyllide and tritium-labeled NADPH, Griffiths and Mapleston [22] detected the formation of tritium-labeled chlorophyllide in preparations of etioplast membranes. This provided direct evidence that NADPH serves as a specific reducing agent during photoenzymatic formation of chlorophyllide in vivo. Based on detailed investigation of Pchlide photoreduction in an artificial active complex composed of protochlorophyllide, NADPH, and barley etioplast membranes, Griffiths introduced the term protochlorophyllide oxidoreductase (POR) for the enzyme catalyzing photoreduction of protochlorophyllide [23].
It was initially supposed that the photoenzyme POR is specific for angiosperms. POR is now known to be a universal photoenzyme occurring in all plant species, from cyanobacteria to higher plants [24-26]. Recent advances in molecular biological methods, including genetic engineering technologies, provided valuable data concerning the structure of the protein counterpart in the active pigment-protein complex of chlorophyll precursor.
The molecular mass of the POR polypeptide, 36 kD, was determined independently by Oliver and Griffiths [27] and Apel et al. [28]. Gel electrophoresis data revealed two POR polypeptides with molecular masses of 35 and 37 kD [29].
POR is encoded in the nucleus and translated to the cytoplasm as a high-molecular-weight precursor (41-44 kD) [30], which is then imported into the plastids through the envelope membranes [30-32]. The transit peptide (about 8 kD) is cleaved off by the stromal peptidase, which gives rise to the enzyme with molecular mass of about 36 kD.
Molecular Structure
The analysis of amino acid sequence [33, 34] and secondary structure of POR [35] showed that this enzyme belongs to the family of short-chain alcohol dehydrogenases within the enzyme superfamily “RED” (reductases - epimerases - dehydrogenases), also known as the SDR superfamily (short-chain dehydrogenases-reductases) [33, 36].
The enzymes of this family are mainly homodimers and homotetramers [37]. They catalyze NADP(H)- and NAD(H)-dependent reactions, including the transfer of hydride-ion and proton. For some members of this enzyme family, the crystal structure was determined [38, 39], and these enzymes were used as a template for the creation of POR homology model [40]. The authors suggested a three-dimensional structural model for the ternary complex Pchlide-NADPH-POR of Synechocystis based on structural homology between POR and the RED superfamily proteins (Fig. 2). Two main features distinguish POR from other members of the short-chain dehydrogenase-reductase family: (i) porphyrin is used as a substrate; (ii) the enzyme activity is strictly light dependent.
The primary structure of POR was first determined from the complementary DNA of barley POR gene cloned in Escherichia coli. The POR sequence contains 388 amino acid residues, and the transit peptide consists of 74 residues [41]. The sequence is characterized by high content of basic amino acid residues and large proportion of hydrophobic amino acids. All POR proteins from higher plants contain four conservative Cys residues (119, 170, 280, and 307), except for the pea POR containing Asp at position 170. In green algae and cyanobacteria, POR has an additional non-conservative cysteine residue. At least one of conservative cysteine residues, probably Cys307, might be involved in Pchlide binding, as indicated by experiments with the use of labeled N-phenylmaleimide [31, 42]. The enzyme active site is bound to C17 propionate group of Pchlide by electrostatic interactions [43].Fig. 2. Model of POR secondary structure [40]. The structure consists of the central parallel beta-sheet comprising seven beta-strands and surrounded with nine alpha-helices. The unique feature of POR compared to other members of RED superfamily is the existence of an external loop (33 amino acid residues) between the fifth and the sixth beta-strands (marked by red color). NADPH is harbored through the N-terminal part of the enzyme molecule.
Secondary Structure of POR
The secondary structure of the enzyme was deduced from circular dichroism spectra in the UV region [35]. The studies on purified monomeric POR have shown the presence in the protein of 33% alpha-helices, 19% beta-sheets, 20% turns, and 28% random coil. The unique property of POR compared to other members of the RED superfamily is the presence of an external hydrophobic loop consisting of 33 amino acid residues between the fifth and the sixth beta-strands. Based on analogy with other enzymes from the group of short-chain alcohol dehydrogenases, whose structure was deduced from X-ray crystal analysis, researchers proposed that protochlorophyll oxidoreductase comprises the central parallel beta-sheet composed of seven beta-strands, which is surrounded by nine alpha-helices [40, 44]. The homology model of POR from Synechocystis was constructed using 7alpha-hydroxysteroid dehydrogenase of E. coli as a structural template (Fig. 2) [40]. The model characteristics correspond to a globular soluble protein whose N-terminal region contains a specific NADPH-binding narrow fold, termed the Rossman fold. Although the precise function of a broad hydrophobic external loop (red line on the figure) is not clear, this loop is thought to ensure Pchlide binding, protein-protein interactions, or the linkage of the enzyme to the membrane (enzyme anchoring) [35].
Comparatively recent studies revealed that active POR in vivo exists in aggregated (dimeric) state [45, 46]. The authors suggest that the dimeric state of POR facilitates the aggregation of Pchlide.
Active Site of POR
In the last few years, considerable efforts were aimed at elucidating the mechanism of Pchlide photoreduction with the use of artificial (reconstituted) ternary complexes comprising constituents of natural complexes (Pchlide, NADPH, and the photoenzyme POR). Analysis of Pchlide photoreduction in reconstituted ternary complexes as a function of the substrate and enzyme concentrations showed that one pigment molecule in vitro is associated with the POR monomer [46, 48]. The quantitative analysis of pigment and protein content in oat etioplasts has shown that the Pchlide/POR ratio (mol/mol) in vivo is also close to unity [49].
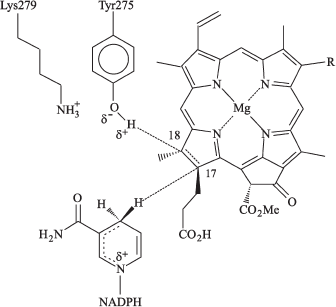
In the family of short-chain alcohol dehydrogenases, the substrate is reduced by hydride-ion supplied from NADPH after its fixation deep in the substrate-binding cleft near the conservative tyrosine and lysine residues involved in proton transfer to the substrate. Using transgenic photosynthetic bacteria Rhodobacter capsulatus with overexpression of pea POR gene, Wilks and Timko [34] observed that the replacement of Tyr275 and Lys279 with Cys and Arg, respectively, led to the loss of the enzyme activity. In this respect, POR differs from the dehydrogenases whose activity was retained after such substitution. Apparently, the porphyrin-binding site in POR is more rigid than the substrate-binding site in dehydrogenases. The authors proposed that tyrosine might act as a proton donor, whereas the lysine residue is crucial for lowering pKa of Tyr275; i.e., it facilitates deprotonation of the tyrosine phenol group. Wilks and Timko suggested the model for the catalytic mechanism of Pchlide photoreduction by oxidoreductase in the active center of the ternary complex (Fig. 3); the model is based on comparison with short-chain alcohol dehydrogenases whose structure is known. According to this model, the position of porphyrin ring D is fixed against NADPH and Tyr275, which optimizes the possibility for hydride-ion and proton transfer. The proton of tyrosine phenol group arrives at the C18 atom of the Pchlide molecule, whereas the hydride-ion is transferred from NADPH to the C17 position.
The role of the conservative lysine residue in POR activity was confirmed by studies on Pchlide photoreduction in reconstituted ternary complexes with POR from Synechocystis sp. [50]. The authors favor the view that the conservative lysine residue is involved in binding of NADPH cofactor.Fig. 3. Suggested model of the catalytic mechanism for protochlorophyllide oxidoreductase in the active center of ternary complex Pchlide-POR-NADPH. Interaction of complex components is shown as in [34].
Lebedev et al. [51] examined photoreduction of protochlorophyllide in reconstituted ternary complexes (Pchlide, POR, and NADPH) by means of spectral methods and directed mutagenesis of pea POR (replacements of Tyr275 and Lys279 with Phe and Ile). The results of this work confirmed the significance of Tyr275 and Lys279 for bringing the pigment-protein complex into the photoactive state; however, these amino acids had no crucial role in the binding of the substrate or the cofactor. The authors suppose that Tyr275 and Lys279 provide for the proper (required for the photoreaction) coordination of NADPH and Pchlide in the enzyme catalytic site and thereby control the efficiency of the formation of the POR photoactive state. Since the replacement of amino acids having polar (Tyr) or charged (Lys) R-group with amino acids bearing nonpolar R-group disturbed spectral properties of the complex and decreased its capacity of formation the photoactive state, the authors emphasize particular significance of POR surface charge in creating optimal conformation for the photoreaction.
Substrate Specificity of POR
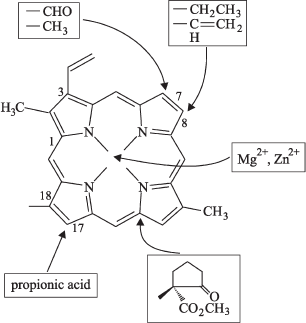
Etiolated plant leaves contain several chemically different chlorophyll precursors: phytol-free protochlorophyllide; protochlorophyll esterified with phytol or its precursors (geranylgeraniol, dihydrogeranylgeraniol, and tetrahydrogeranylgeraniol), as well as mono- and divinyl forms of these pigments. These pigment forms differ in photochemical activity depending on their ability to interact with the POR enzyme. The specificity of POR to the substrate in vitro was investigated in experiments with isolated enzyme. It turned out that the protochlorophyll(ide)-POR binding and photochemical activity of the complex depend critically on certain atomic groups in the pigment molecule. Klement et al. [49] examined photoreduction of Pchlide derivatives in artificial complexes with NADPH and POR in order to assess the substrate specificity of POR. They analyzed 13 protochlorophyll(ide) analogs. Six of these analogs having various side groups in rings A and B behaved as active substrates, whereas other analogs with substituted side chains in rings D and E lost their capacity of being the POR substrate. It was found that at least three sites of the pigment molecule play the key role in the pigment binding to the enzyme active site, namely, the central metal atom, propionic acid (in the side chain of ring D), and the ring E structure.
Based on various data concerning the protochlorophyll(ide) molecule, Schoefs and Franck [52] presented a schematic view of its chemical features essential for the pigment molecule to act as a POR substrate (Fig. 4).
It turned out that the isolated POR is able to reduce protochlorophyllide a but not protochlorophyll (esterified pigment form) [20, 43, 53, 54]. The activity of the complex depended crucially on the size and the nature of the side group at C17 position. This group should be a propionic acid holding a free carboxylic group at C17 position. Longer alcohol chains like phytol or an acrylic group prevent the photoreduction of the pigment [49, 55]. The replacement of side-chain propionate in ring D with methyl ester of propionic acid or with acrylate diminished the photochemical activity [49]. The ionic bond between acrylate and the enzyme is still possible, but this bond is more rigid than the bond with propionate. However, there are some exceptions from this rule. For example, the POR isolated from C-2A mutant of Scenedesmus obliquus green alga was competent in reducing protochlorophyllide esterified by long-chain alcohol [56]. Ignatov and Litvin studied photoreduction of esterified long-wavelength protochlorophyll form (Pchl 682/672) in mutant chlorella cells and found that this protochlorophyll form is capable of conversion to the long-wavelength chlorophyll form [57, 58]. The authors supposed that algae probably contain a specific POR form that catalyzes photoreduction of esterified molecule of chlorophyll precursor. Remarkably, some evidence that esterified protochlorophyllide is able to produce chlorophyll was obtained in earlier studies [59-67], but the efficiency of this transformation was very low.Fig. 4. Principal positions at porphyrin ring of protochlorophyllide molecule required for POR activity. Frames indicate chemical groups responsible for the POR-mediated photoreduction of substrate [52].
Both mono- and divinyl protochlorophyllide forms can act as the POR substrate. The side group at the C8 position can be either vinyl (DV-Pchlide) or ethyl (MV-Pchlide) [68-70]. The side group at the C7 position can be either methyl, as it occurs in protochlorophyllide a, or aldehyde as in protochlorophyllide b [54]. However, the existence of protochlorophyllide b in etiolated plant leaves is the subject of controversy.
The central Mg2+ atom plays an important role in Pchlide binding to POR. The replacement of Mg2+ with other metals (except for Zn2+) leads to the loss of the complex activity. Upon the replacement of magnesium with zinc, the ternary complex retained its activity [43, 71, 72].
The activity of ternary complex depends largely on the structure and steric configuration of ring E (the enzyme-binding site) in the Pchlide molecule [49]. Protochlorophyllide with chemical modifications of ring E at positions C131 and C132 is unable to act as the POR substrate [49]. Alteration in stoichiometry of -H and -CO2CH3 groups at the position C132 [49, 73] also inhibits the Pchlide photoreduction. These pigment modifications eliminated the ability to produce enolates by means of keto-enol tautomerization. Based on these data, the authors concluded that the formation of enolates through keto-enol tautomerization is necessary for the photoreaction. Thus, protochlorophyllide a´ (a stereoisomer at the position C132) cannot be used as a POR substrate. Hence, the chlorophyll a´ known to function in the reaction center of PSI should apparently derive from chlorophyll rather than from protochlorophyll [74].
Considering the assumption that the Rossman fold in the POR protein represents the active site, like in homological alcohol dehydrogenases [34], the fixation of rings D and E at the bottom of this fold is consistent with experimental data obtained in [49].
HETEROGENEITY OF PROTOCHLOROPHYLLIDE OXIDOREDUCTASE
As mentioned above, Oliver and Griffiths [29] investigated POR from pea and bean plants with gel electrophoresis and observed two bands containing polypeptides with molecular masses of 35 and 37 kD. However, for a series of plant species, only a single POR polypeptide was found in early studies. According to various data, the POR size ranged in different species from 33 to 38 kD [29, 30, 75, 77-79]. The application of isoelectric focusing revealed the existence of at least four different species of protochlorophyllide reductase [76, 80].
In 1995 researchers from Apel's laboratory reported that Arabidopsis thaliana and barley contain two types of nuclear genes coding for POR: PorA and PorB (with 75% homology) [81, 82]. In consistency with this, two corresponding POR forms were termed PORA and PORB. The molecular mass for PORB (37 kD) is somewhat higher than for PORA [83]. The biochemical activity of PORA and PORB was demonstrated in vivo using transgenic plants with overexpression of these enzymes [84-87].
The expression of the PorA gene was observed in etiolated seedlings; hence, PORA is synthesized in darkness and is the main constituent of paracrystalline prolamellar bodies in etioplasts. However, the transcription of PorA gene ceases in the light, and the enzyme degrades rapidly (during the first 4 h of greening) under the action of light-induced protease [88-90]. On the other hand, the transcription of PorB gene occurs both in darkness and in the light, while the subsequent continuous translation produces the enzyme responsible for biosynthesis and accumulation of chlorophyll under daylight illumination. The transport of cytoplasmic PORA precursor into the plastids is a protochlorophyllide-dependent process, whereas the translocation of PORB precursor is not [85]. Analysis of POR gene expression in light-grown arabidopsis and barley plants as a function of their age has shown that PorA is expressed almost exclusively in young seedlings, while the expression of PorB occurs both in seedlings and mature plants [81, 91]. Since etiolation and formation of prolamellar bodies is a part of the natural diurnal dark-light cycles, one may expect that PORA and PORB play a certain role during biosynthesis and accumulation of chlorophyll a under natural photoperiodic conditions. The formation of etioplast prolamellar bodies and the active form of protochlorophyllide, Pchlide 655/650 is related to the presence of POR. The role of two POR varieties in the formation of photoactive structures was investigated using A. thaliana mutants deficient in prolamellar bodies and Pchlide 655/650 after overexpression of both POR genes [85]. The results proved that restoration of both PORA and PORB gives rise to formation of etioplasts with large prolamellar bodies and elevates the content of photoactive Pchlide 655/650. The authors concluded that both enzymes (PORA and PORB) are responsible for etioplast differentiation.
The number of POR genes differs in various plant species. In prokaryotes, only PORB was observed, apart from the structurally unbound enzyme catalyzing protochlorophyllide reduction in darkness. In seed plants (Spermatophyta), PORA is acquired in addition to PORB. In A. thaliana, only PorA and PorB genes were initially observed, while further studies revealed the existence of the third gene PorC (see below). The leaves of loblolly pine (Pinus taeda) were found to contain two families of Por genes: the porA family includes two genes, while the porB family comprises at least 11 genes [92]. The appearance of more than one gene is not a common rule. Only one gene was observed in some cyanobacteria [93, 94], green algae [95], cucumber green leaves [96], and pea [97].
Recent studies unveiled a third POR variety. During sequencing of the A. thaliana genome, a gene was identified that encoded a new, previously unknown POR-like protein [98, 99]; this protein was named PORC. The molecular mass of this protein is 38 kD [83]. Unlike PorA and PorB, the PorC gene was not expressed in etiolated leaves; the accumulation of the respective mRNA started immediately after the onset of illumination [98, 99]. Similar results were obtained earlier for POR of cucumber [100] and Marchantia [101]. Regarding the functional role of PORC, the question arose of whether this enzyme is redundant or whether it facilitates plant adaptation by adjusting chlorophyll biosynthesis to various light conditions. After expressing the PorC gene in E. coli, the activity of PORC was detected in vitro [99]. It was found that PORC activity (similarly to PORA and PORB activities) depends on availability of light and NADPH. In mature light-adapted plants, only mRNA of PORB and PORC were identified. The difference between PORB and PORC was observed in seedlings grown under continuous white light. After the transfer of such plants to darkness, the content of PORC mRNA decreased rapidly to indiscernible level, while the content of PORB mRNA remained unchanged. Upon the exposure of plants to light of various intensities, the amount of PORB mRNA remained constant, while the content of PORA and PORC mRNA varied depending on light intensity. Appreciable amounts of PORA mRNA were only observed in seedlings grown under low-intensity light and were absent at high irradiance, whereas the content of PORC mRNA increased with light intensity. These differential responses to light led to the suggestion that A. thaliana possesses all three functional POR forms. These forms allow the plant to meet demands in chlorophyll biosynthesis in a selective manner through preferential use of one of three enzymes under different light regimes. These conclusions were substantiated by studies on A. thaliana mutants deficient in synthesis of PORB or PORC [83, 102]. In etiolated seedlings of the porB mutant, the protochlorophyllide photoactivity was comparatively low and prolamellar bodies had small dimensions. When exposed to light, etiolated seedlings of porB mutant synthesized chlorophyll to the same extent as the wild type plants; however, the capacity of chlorophyll synthesis decreased markedly under low-intensity illumination. The greening of mutant plants deficient in PORC was suppressed with the increase in light intensity. This observation led to the conclusion that accumulation of PORC during greening at high irradiances protects etiolated seedlings against damaging action of light. The authors supposed that PORB also performs the protective function during greening, because etiolated seedlings of porB mutants were more sensitive to inhibition of greening by far-red light than the wild type seedlings.
Thus, it is presently established that plant leaves contain three forms of protochlorophyllide oxidoreductase (PORA, PORB, and PORC) encoded by different genes. These proteins are quite similar in structure, as evidenced by high homology of their amino acid sequences [98] and similar molecular masses (36, 37, and 38 kD, respectively).
At the same time, the synthesis of three POR species, like synthesis of corresponding mRNA, is differentially regulated by light. The transcription of PorA is strongly inhibited by light; transcription of PorB is stimulated by light during de-etiolation but is insensitive to irradiance upon growing plants under continuous white light [81, 82]. The transcription of PorC was indiscernible in darkness, but it increased during illumination and was stimulated by high-intensity light [98, 99, 102].
It is remarkable that organisms having only one POR gene exhibit various dependences of enzyme transcription on light conditions. Depending on plant species, light either has no effect [97, 103] or stimulates [96, 100] or inhibits [104] the POR transcription. Further studies of photoregulated biosynthesis of POR and protochlorophyllide might clarify the origin of these distinctions.
LOCALIZATION OF ACTIVE COMPLEX IN PLASTIDS AND ASSOCIATION OF
PHOTOENZYME WITH MEMBRANES
The first attempt to elucidate the localization of protochlorophyllide was undertaken by Boardman and Anderson (1964) with the use of fluorescent microscopy [105]. These authors discovered that fluorescence in etioplasts is distributed not evenly but concentrates in a limited region, which they called the “stroma center”. The ultrastructure of etioplasts was investigated by means of electron microscopy [106, 107]. These studies revealed tubular membranes arranged in a paracrystalline structure, which were termed “prolamellar bodies” (PLB). Since that time the “stroma center” was renamed to PLB.
The lamellar membranes surrounding PLB were named “prothylakoids” (PT). Later studies based on light spectroscopy [108, 109], biochemical analyses [110], and immunoenzyme assays [111, 112] showed that POR and the active protochlorophyllide form Pchlide 655/650 are mainly localized in prolamellar bodies, although their minor quantities are also detected in membranes of stromal prothylakoids. Interestingly, the POR of prothylakoids turned out more resistant to photodestruction compared to POR located in PLB [111]. Other authors confirmed later the correlation between the POR content and PLB formation [87]. However, in young (2- or 3-day-old) etiolated leaves, where prolamellar bodies were not yet formed [113], the active protochlorophyllide form was concentrated in prothylakoids.
The photoenzyme POR is not an integral membrane protein; it can be classified as a peripheral membrane protein located on the stromal thylakoid side, because the POR hydrophobic part contains no apparent region sufficiently long for spanning the membrane [24, 31, 79, 97, 114-116]. The peripheral nature of POR is also supported by its solubilization at low concentrations of detergent (n-octyl-beta-D-glucoside), which is typical of exposed membrane proteins [49].
Immunological analysis of chloroplasts from light-adapted plants and green algae has shown that POR and Pchlide are localized on the stromal side of thylakoid membranes and in the chloroplast envelope membrane [117-121]. The activity of POR in protochlorophyllide photoreduction was observed under illumination of chloroplast envelope membranes in the presence of NADPH [118, 122]. Dahlin et al. [119] investigated POR import into pea chloroplasts in vitro and concluded that POR attaches to the stromal side of thylakoid membranes as a peripheral protein. The distribution of POR between stromal thylakoids and the inner membranes of chloroplast envelopes appears to depend on the plant species [120].
Association of POR with Membranes
Analysis of the secondary structure of POR [35] suggested that POR binds to the membrane via the amphiphilic segment located in the C-terminal protein region containing a tryptophan residue. Alternatively, the binding might involve a long extra loop located in the region responsible for enzyme “anchoring” [35]. Aronsson et al. [123, 124] examined pea mutants with deletions in the C-terminus of POR and showed that amino acids of this region (starting from the 362nd residue) are indispensable for the binding of POR to the membrane and, apparently, it is the C-terminus that anchors POR at the membrane.
Already in 1993, Teakle and Griffiths put forward a proposal on the electrostatic nature of bonds between POR and the membrane [31]. In the last few years, a series of reports appeared concerning significance of surface charge attributed to POR amino acid residues for POR binding to thylakoids [51, 125]. The replacement of charged amino acids with neutral alanine by means of mutagenesis [125] showed that the amino acid substitution in the protein central part (between the 86th and 342nd residues) eliminates the proper attachment of POR to thylakoids and decreases its catalytic activity.
Thus, the POR molecule is fixed on the membrane with its C-terminal domain, but the position required for catalytic function is controlled by the surface charge of amino acids in the central part of the enzyme.
MULTIPLICITY OF CHLOROPHYLL PRECURSOR FORMS in vivo
Heterogeneity of active pigment-enzyme complexes of the chlorophyll precursor is also indicated by the presence in the active complex of various pigment forms differing either in molecule chemical structure (as detected with extracted pigments) or in the state or condition of pigments in the total complex. These distinctions become evident during spectral investigations of whole cells or isolated complexes.
Chemically Different Forms of Chlorophyll Precursor
Protochlorophyll and chlorophyll. As stated above (see subsection “Substrate specificity of POR”), the active complex of chlorophyll precursor includes mainly the phytol-free pigment form, protochlorophyllide. Already Godnev et al. [126] put forward the idea that the phytol chain substantially hinders the binding of the esterified molecule to the specific protein carrier. Griffiths [43] demonstrated the crucial significance of free carboxylic group at the 17th carbon atom for the formation of pigment-protein complex. After esterification of the propionate residue even by the small methyl group, the pigment lost its capacity of binding to the protein. At the same time, it was frequently reported that phytol-esterified molecules of chlorophyll precursor are competent in photochemical reduction leading to chlorophyll formation, even though the efficiency of this reaction is low [59, 61, 66, 67, 127-131]. Therefore, it is reasonable to suppose the existence of a minor pool of active complexes comprising the phytol-esterified form of chlorophyll precursor. Interestingly, the esterified protochlorophyll is mainly located in prothylakoids [132].
The application of mass spectrometry and gas-liquid chromatography made it clear that the esterifying alcohols can be also represented by phytol precursors, namely, geranylgeraniol (GG; C20H33OH), dihydrogeranylgeraniol (C20H35OH), and tetrahydrogeranylgeraniol (C20H37OH) [133-137]. Protochlorophylls esterified by various alcohols, known as phytol precursors, were isolated from etiolated seedlings of various plant species [136, 137]. In barley leaves, a part of GG-protochlorophyll can be converted by light into GG-chlorophyll a [135].
Monovinyl- and divinyl protochlorophyll(ide). Several groups of authors [64-66, 138-141] established that both pools of chlorophyll precursor (i.e. protochlorophyllide and its esterified form) are heterogeneous: they comprise both monovinyl- and divinyl-modifications of the chromophore (not only monovinyl-protochlorophyllide, as was supposed in earlier studies). These modifications differ in the location of side vinyl groups in the molecule macrocycle. Monovinyl-protochlorophyllide contains the vinyl group at the third carbon atom in pyrrole ring A, whereas divinyl-protochlorophyllide contains two side vinyl groups at the third and eighth atoms in pyrrole rings A and B. In the molecule of monovinyl-Pchlide, the second vinyl group at position 8 in the macrocycle is replaced with an ethyl group. Mono- and divinyl-protochlorophyll(ide)s were separated by means of thin-layer chromatography.
Mono- and divinyl forms of protochlorophyll(ide) differ in absorption spectra (or excitation spectra of fluorescence emission) in the blue spectral region (in the Soret band). The monovinyl protochlorophyll dissolved in ether at 77K exhibits the absorption peak at 437 nm (with a minor satellite band at 443 nm); the divinyl-protochlorophyll form has an absorption maximum at 443 nm (with a shoulder at 451 nm) [64-66, 140]. Under the action of light, both protochlorophyllide forms are converted to different chlorophyllide forms differing in the position of absorption bands in the blue spectral region. Then, monovinyl- and divinyl-chlorophyllide are converted in parallel dark reactions into corresponding chlorophyll a forms that also differ in the positions of the short-wavelength absorption bands [142].
The relative contribution of monovinyl- and divinyl-components to the total pool of protochlorophyllous pigments depends on the taxonomical classification of a given plant species. Etiolated monocotyledonous plants accumulate mainly monovinyl forms of the chlorophyll precursor [65, 143]. Divinyl protochlophyll(ide)s are predominant in dicotyledonous species [143, 144]. Some dicotyledons (cucumber, beans) produce both varieties of protochlorophyllous pigments in approximately equal amounts [143].
Twenty or thirthy years ago, many researchers believed that divinyl pigments are the precursors of monovinyl forms [131, 139, 145]. However, detailed investigations of Rebeiz and coauthors [64-67, 140, 141, 146, 148, 149] made it clear that both components of the chlorophyll precursor pool in higher plants are synthesized independently. The dark biosynthesis of the precursor is realized through four parallel pathways (the branching occurs at the stage corpoporphyrinogen) and results in accumulation of monovinyl- and divinyl-derivatives of Pchlide and protochlorophyll, which are reduced in the light, resepectively, to monovinyl- and divinyl-chlorophyllide and chlorophyll. Next, divinyl-chlorophyll is reduced to monovinyl-chlorophyll. This reaction is catalyzed by 8-vinyl reductase. In higher plants, all functional chlorophyll belongs to the monovinyl form. Later, Rebeiz et al. suggested even more complicated scheme [150] containing six parallel pathways for the formation of chlorophyll precursor molecule. Two additional pathways take their origin from divinyl- and monovinyl-protoporphyrinogen esterified by the alkyl group with unknown carbon-chain length.
Both protochlorophyllide pools occurring in etiolated plants--the active pool (converted to chlorophyllide under the action of light) and inactive pool--represent the mixture of divinyl- and monovinyl-pigment forms in approximately equal proportion [87]. The view was proposed that chemically different chlorophylls synthesized from different precursor forms might perform some specific roles in photosynthesis [149]; however, this hypothesis has not been confirmed so far.
Thus, we can reasonably conclude that etiolated plant leaves contain four main chemical varieties of chlorophyll precursor, namely, protochlorophyll esterified with phytol at the 17th position, Pchlide, as well as their divinyl- and monovinyl-forms. The active precursor pool consists mainly of phytol-free Pchlide comprising divinyl- and monovinyl-forms in the proportions determined by taxonomical position of the plant.
Spectrally Different Forms of the Chlorophyll Precursor
Absorption spectra of protochlorophyll(ide) in vivo and in vitro are substantially different. The main red maximum of protochlorophyll is observed at 621-623 nm for ether as a solvent and at 633-640 nm in pyridine [151, 152]. Under natural condition, i.e. in the intact etiolated leaf, the red band of protochlorophyll absorption was observed at 650 nm [151-154]. However, water homogenates of etiolated leaf substance (colloid solutions) exhibited the absorption peak at 635-636 nm [9, 10].
In the late 1950s, Shibata applied a special technique of absorption measurements to light-scattering objects and was the first to detect two red maxima in the absorption spectra of intact etiolated leaves at 636 and 650 nm [155, 156]. Shibata assigned these peaks to different forms of the chlorophyll precursor: the long-wavelength form capable of photoconversion to chlorophyll (Pchlide 650) and the inactive short-wavelength form. However, later studies revealed that the protochlorophyll form having absorption band at 636 nm is also capable of transformation to chlorophyll(ide), although the activity of this form is lower than that of Pchlide 650.
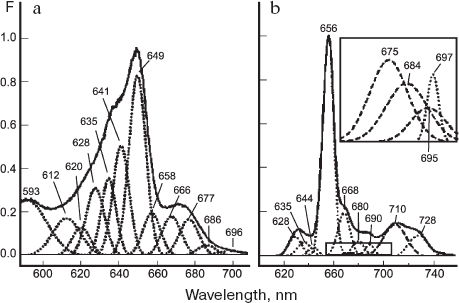
Spectra of protochlorophyllide fluorescence in vivo were successfully measured owing to deep-freezing of plant material with liquid nitrogen, which retarded phototransformations of Pchlide. This approach, first applied by Litvin and Krasnovsky [157-159] for studying pigments in etiolated and greening leaves, became a widely used tool for exploration of properties and functions of all photosynthetic pigments. The low-temperature fluorescence spectra of etiolated leaves were found to contain the main maximum at 655 nm and a small maximum at 633 nm. In the long-wavelength region, small emission peaks were recorded at 690 and 705-707 nm. Subsequent studies of fluorescence spectra in etiolated leaves revealed in addition a few smaller maxima in the red spectral region. Six registered bands were located at 629-635, 655-657, 674, 686-690, 712-713, and 725-728 nm [160-165].
The application of derivative spectroscopy methods and mathematical deconvolution of spectra into individual Gaussian components led to identification of a few additional bands in the absorption and fluorescence spectra of etiolated leaves [166]. The bands at 669, 676, 686, 696, and 710 nm were observed in the long-wavelength region of absorption spectrum and the peaks at 669, 686, 720-712, and 728 nm were detected in the fluorescence spectra. The authors concluded that only one band around 712 nm represents the vibrational satellite of the main band at 656 nm. The decomposition of spectra into a sum of Gaussian components showed that the full assortment of protochlorophyll forms in vivo is the following: P633/628, P642/637, P655/650, P669/657, P682/669, P692/675, P?/685, P?/697, and P730/711 (the slash-separated numbers represent the fluorescence and absorption maxima, respectively). In later studies of other researchers, the decomposition of spectral curves into individual Gaussian components was applied for characterizing the number and parameters of spectrally different protochlorophyll forms. The results obtained in these studies [132, 167-169] were basically consistent with the previous data. Investigation of Pchlide spectral forms in etiolated leaves of 12 plant species by means of decomposition of fluorescence spectra into Gaussian components allowed Boddi et al. [167] to infer the existence in taxonomically various etiolated plants of four universal forms of protochlorophyllide, characterized by fluorescence bands at 633, 645, 657, and 670 nm.
Figure 5 shows recent results [170] of a complex spectral investigation of etiolated leaves from various plant species (including mutants). Etiolated leaves of these species, characterized by various proportions of individual spectral forms, were examined on the basis of their fluorescence spectra, fluorescence excitation spectra, and the respective derivative spectra. The spectral curves were decomposed into constituent Gaussian components. The authors succeeded in distinguishing vibrational satellites of the main Pchlide forms from the long-wavelength bands attributed to individual Pchlide forms. In addition to vibrational satellites in the long-wavelength region (at 675, 684, 695, and 712 nm), five fluorescence bands were revealed and assigned to different protochlorophyllide forms (at 666, 680, 690, 698, and 728 nm); the respective bands in the fluorescence excitation spectra were at 658, 668, 677, 686, and 696 nm. Based on these data, the list of protochlorophyllide forms and their spectral parameters were ascertained. In comparison with previous work [166], additional Pchlide forms--Pchlide 627/620 and Pchlide 646/640--were identified in the short-wavelength region. The observed correspondence between the absorption bands at 686 and 696 nm and fluorescence peaks at 697 and 728 nm led to the recognition of Pchlide 697/686 and Pchlide 728/696 forms that remained unidentified in previous studies.
Thus, etiolated leaves contain three main spectral forms of protochlorophyllide (Pchlide 633/628, Pchlide 643/637, and a dominant form Pchlide 655/650), as well as five minor long-wavelength forms (Pchlide 666-669/658, Pchlide 680-682/668, Pchlide 690-692/677, Pchlide 698/686, and Pchlide 728/696).Fig. 5. Decomposition of low-temperature fluorescence spectra (b) and fluorescence excitation spectra (a) of etiolated maize leaves into Gaussian components. Dotted lines designate electronic transitions; dashed lines designate vibrational components. Spectra of fluorescence excitation were measured for the emission at 740 nm [170].
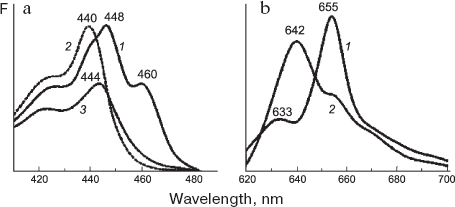
Main spectral forms of protochlorophyllide. Three main spectral forms of chlorophyll precursor are manifested in different manners in the absorption and fluorescence spectra. The band at 628 nm in the absorption spectrum of normal etiolated leaf is either unresolved (owing to its superposition with neighboring absorption bands) or is evident as a very weak shoulder. Since the short-wavelength Pchlide 633/628 form is characterized by very weak photoactivity at room temperature and is incapable of phototransformation at freezing temperatures, its absorption band at 628 nm turns clearly visible in the absorption spectrum of light-exposed etiolated leaf after the disappearance of two active Pchlide forms. The band at 628 nm appears as a small peak in the derivative absorption and fluorescence excitation spectra; it is also evident upon the decomposition of these spectra into constituent Gaussian components. In the short-wavelength absorption region of Pchlide 633/628, the main peak is positioned at 440 nm (Fig. 6).
The short-wavelength form Pchlide 628/633 showing no transformation during short-term illumination is considered inactive [167, 171]. However, in plants enriched with this form, this Pchlide was slowly transformed into chlorophyllide [172-175]. Our studies demonstrated that this form is capable of conversion to chlorophyll only at temperatures above 5°C [176]. Some evidence indicates that the short-wavelength Pchlide form is the precursor of the main photoactive form Pchlide 655/650 in the dark pathway of pigment synthesis [21, 177, 178]. Specifically, Griffiths [21] showed by means of differential spectroscopy that a 10-min incubation of isolated prolamellar bodies from barley etioplasts in the presence of NADPH at room temperature after preillumination ensured the photoconversion of active protochlorophyllide form Pchlide 655/650 and was accompanied by the decrease in the content of Pchlide 633/628 with the parallel appearance of long-wavelength forms absorbing at 640 and 650 nm. Analysis of action spectra for chlorophyll formation also indicated that the inactive short-wavelength form, Pchlide 633/628 participates in this process through its conversion to the main long-wavelength photoactive form [179]. The Pchlide 633/628 form was thought to represent the protochlorophyllide lacking specific link to POR [23, 42, 132]. On the other hand, it is not excluded that the protochlorophyllide corresponding to the short-wavelength form is bound to POR in such a way that its activity is lost [49]. Supporting evidence in favor of Pchlide 633/628 binding to protein comes from the fluorescence lifetime of this Pchlide form. According to a recent report [180], the fluorescence decay (decay time of about 6 nsec) is significantly faster that the fluorescence decay time for protochlorophyllide solutions (around 10 nsec).Fig. 6. Low-temperature (77 K) spectra of (a) fluorescence excitation and (b) fluorescence emission for the main protochlorophyllide forms that were measured on 7-day-old etiolated bean leaves [194]. a) Fluorescence was recorded at 655 (1), 633 (2), and 642 nm (3); spectrum 3 was measured with the etiolated bean leaf incubated for two days in the solution of delta-aminolevulinic acid with the purpose of accumulating the Pchlide 643/639 form. b: 1) Fluorescence spectrum for the 7-day-old etiolated bean leaf; 2) spectrum of etiolated leaf incubated for two days in darkness in the presence of 10 mM delta-aminolevulinic acid with the purpose of accumulating the Pchlide 643/639 form.
The protochlorophyllide form Pchlide 643/637 is observed in absorption spectra (main peaks at 637 and 444 nm) but is virtually indiscernible in fluorescence spectra. This results from highly efficient (nearly 100%) excitation energy transfer from this form to Pchlide 655/650 [165, 181-183]. The band at 643 nm becomes apparent in the spectrum of etiolated leaves after some treatments (including the isolation of active pigment-protein complexes) that disturb their native condition and the effective energy transfer between pigment molecules. This form accumulates in large quantities during incubation of etiolated leaves in the solution of ä-aminolevulinic acid (see Fig. 6). The Pchlide 643/637 is the dominant form in etiolated leaves of some plant species [184, 185]. In the subtracted fluorescence spectra “light - minus - dark” [169], the negative maximum at 643-644 nm was only observed in very young (before the fourth day) etiolated leaves. The exposure of plants in darkness for longer periods enhanced the excitation energy transfer form this Pchlide form to the main long-wavelength Pchlide 655/650. The Pchlide 643/637 form is photochemically active; under the action of light it is converted to chlorophyllide, even at rather low temperatures, similarly to protochlorophyllide Pchlide 655/650. However, its photochemical activity in vivo depends more strongly on temperature compared to the photoactivity of the main active form, Pchlide 655/650 [176].
The main photoactive form, Pchlide 655/650, is only observed in whole etiolated leaves. The short-wavelength region of its absorption spectrum contains two bands at 448-450 and 460-462 nm (see Fig. 6). Upon the disruption of the leaf native state, the form Pchlide 655/650 disappears concurrently with the increase in absorbance and fluorescence of short-wavelength forms. The long-wavelength peak position for active protochlorophyllide forms, Pchlide 643/637 and Pchlide 655/650 results apparently from binding of the chromophore to the enzyme POR and hydrogen donor NADPH, as well as from chromophore-chromophore interactions of pigment molecules. This inference is supported by investigations of circular dichroism [186, 187], fluorescence polarization, and excitation energy transfer [188]. The molecules of Pchlide are thought to produce dimers [186] or tetramers [188]. The pigment-pigment interaction is possibly due to aggregation of chromophore-bearing protein molecules (POR).
The state of cofactor NADPH seems also to affect the spectral properties of active Pchlide form. The addition of NADP+ to illuminated preparations of etioplasts or etioplast membranes (light treatment converts the active protochlorophyllide form to chlorophyll) led to accumulation in the subsequent dark period of an inactive pigment form with the absorption maximum at 642 nm and the fluorescence peak at 649 nm, whereas the addition of NADPH in darkness resulted in accumulation of long-wavelength photoactive form of protochlorophyllide with the absorption maximum at 650 nm and the fluorescence maximum at 655-657 nm [189-191].
Furthermore, a substantial role in production of the long-wavelength form Pchlide 655/650 in vivo apparently belongs to lipids of etioplast inner membranes that are thought to promote the aggregation of pigment-protein complexes (see below).
The protochlorophyllide Pchlide 655/650 transforms to chlorophyllide under the action of light even at very low temperatures (down to 190 K).
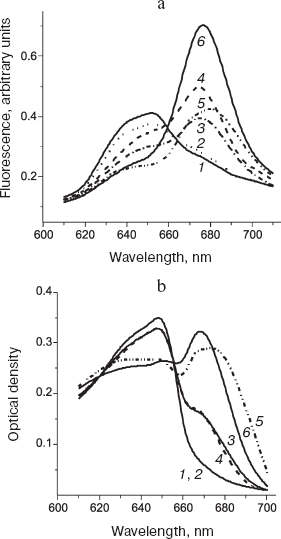
Two pools of active protochlorophyllide form, Pchlide 655/650. In our studies [192, 193], we proposed the existence of two pools of the photoactive protochlorophyllide form, Pchlide 655/650. This view was based on observations that a short-term illumination of etiolated leaves at low temperature resulted in parallel formation of two primary chlorophyllide forms. Recent studies provided direct evidence for the existence of two pools of Pchlide 655/650 [169, 194, 195]. When the subtracted “light-minus-dark” fluorescence spectrum was decomposed into constituent Gaussian components, it became evident that the fluorescence band of photoactive Pchlide 655/650 forms comprises two components with maxima at 652-653 and 657 nm [169]. The highest content of Pchlide 653 form accumulated in very young (2- or 3-day-old) etiolated leaves, when the prolamellar bodies were not yet formed. The accumulation of photoactive Pchlide with the fluorescence maximum at 653 nm was also observed in leaves of plants grown under normal photoperiodic (day/night) conditions after a preliminary dark period [169], as well as in greening barley leaves exposed to light for a few hours, when the prolamellar bodies underwent destruction while the thylakoids developed [174]. Based on these facts, the authors supposed that the protochlorophyllide Pchlide 653 is bound to prothylakoids or thylakoids, unlike Pchlide 655 that is bound to PLB. Ignatov and Litvin [194, 195] also showed that the main active form, Pchlide 655/650 exists in two modifications having fluorescence maxima at 653 and 655 nm and absorption peaks at 648 and 650 nm. The absorption spectra of these two forms differ substantially in the blue spectral range: the short-wavelength bands of these forms are positioned at 440 and 450 nm, respectively. It was proven that the short-wavelength form of chlorophyll precursor, Pchlide 653/648, accumulates in very young seedlings and acts as a precursor for nonfluorescent chlorophyll P680 of PS2 reaction centers [194, 195].
The main forms of chlorophyll precursor under discussion are found not only in etiolated leaves and at early stages of greening [169, 196] but also in fully formed green leaves [197, 198] (Fig. 7) and in dark grown seedlings of gymnosperms [199].
Photoconversions of these forms observed on subsequent illumination provide evidence that the ongoing chlorophyll synthesis in green plant leaves proceeds via the same precursor forms as it occurs in etiolated leaves [197, 198] (see Fig. 7).
Fluorescence-based studies of chlorophyll precursor forms in whole leaves under physiological conditions. Advances in studying the photochemical stages of chlorophyll biosynthesis in whole cells became possible owing to sensitive methods of fluorescence measurements. The obstacle inherent to fluorescence technique was that the light required for fluorescence excitation induced also the photoconversion of pigments. This problem was circumvented by deep freezing of the object (see above, section “Spectrally Different Forms of the Chlorophyll Precursor”). However, this approach had also some serious drawbacks, because the fluorescence measurements cannot be accomplished on the same material, and because fluorescence characteristics during the process occurring at physiological temperature remained unknown.Fig. 7. Accumulation and photoconversion of main protochlorophyllide forms in Hibiscus green leaves [198]. Spectra: 1, 2) low-temperature (77 K) fluorescence spectra of green leaves that were measured before and after 16-h incubation in darkness; 3) after additional irradiation with white light, 103 W/m2; 4) after second additional irradiation for 10 min with white light, 103 W/m2.
Quite recently, the sensitivity of the method was considerably improved in our laboratory (Dubrovskii and Litvin, unpublished data), which opened way to circumvent the aforementioned restriction. It became feasible to measure fluorescence at very low irradiances, at which the photochemical action of light was virtually absent (Figs. 8 and 9). Owing to this technique, the photochemical stage of the process was successfully investigated under physiological conditions in the same sample during the pigment conversions.
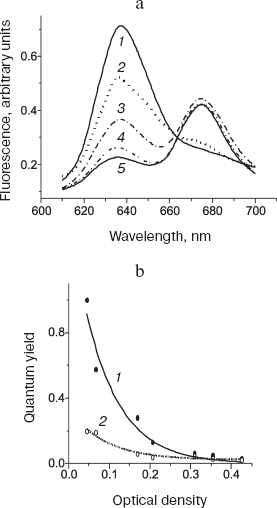
Fig. 8. Fluorescence of short-wavelength form of the chlorophyll precursor in bean leaves under physiological conditions (25°C). a) Fluorescence spectra (excitation wavelength 445 nm) that were measured on the same leaf: 1) initial spectrum of unfolded etiolated leaf; 2) the same as 1 after illumination for 2 min at irradiance 6.7 mW/m2; 3) the same as 2 after additional illumination for 5 min at irradiance 200 mW/m2; 4) the same as 3 plus 2-sec illumination at irradiance of 100 W/m2; 5) the same as 4 after 15-min incubation in darkness. b) Changes in the fluorescence quantum yield during accumulation of the precursor in etiolated leaves. The abscissa axis corresponds to the absorbance in the red maximum. The ordinate axis shows the relative quantum yield of fluorescence at 638 (1) and 655 nm (2).
The authors examined fluorescence of the chlorophyll precursor active forms starting from the earliest developmental stages of etiolated seedling (plants with yet unopened cotyledons), when the dominant pigment form is a short-wavelength protochlorophyllide with fluorescence maximum at 637-638 nm (Pchlide 638). It is seen in Fig. 8a that the low-intensity light converts the Pchlide 638 form to the product whose spectrum is characteristic of chlorophyll(ide). The additional high-intensity illumination initially increased fluorescence of the reaction product. However, at longer and more intense irradiation the fluorescence attained a certain limit, which can be explained as a consequence of excitation energy transfer to the photoproduct (this process competes with the photoreaction). Remarkably, the fluorescence spectrum of the produced chlorophyllide forms did not shift to the long-wavelength region at very high irradiance (curves 3 and 4), although such shift was observed for the main precursor form having the fluorescence maximum at 655 nm. This fact indicates that the short-wavelength precursor form, Pchlide 638, participates only in one of two sequential photoreactions of the chlorophyll formation pathway. Figure 8b shows changes in the fluorescence quantum yield for the short-wavelength (638 nm) and the dominant (655 nm) precursor forms that develop during accumulation of protochlorophyllide in etiolated seedlings. The relative quantum yield of fluorescence for the short-wavelength form decreased exponentially as the pigment accumulation progressed. The fluorescence yield of the long-wavelength form was substantially lower and changed insignificantly during the accumulation of this form. This observation is consistent with the inference of excitation energy transfer, with the donor and acceptor represented by the short-wavelength (Pchlide 638) and the long-wavelength forms, respectively.Fig. 9. Comparison of fluorescence spectra (a) and absorption spectra (b) during photoconversion of the main chlorophyll precursor form (Pchlide 655/650). Spectra were measured under physiological conditions (25°C) on the same leaves (6-day-old etiolated bean seedlings). 1) Initial spectrum of etiolated leaf; 2) the same as 1 after 2-min illumination at irradiance 6.7 mW/m2; 3) the same as 2 after additional 5-min illumination at irradiance 200 mW/m2; 4) the same as 3 after 15-min incubation in darkness; 5) the same as 4 plus 2-sec illumination at irradiance 100 W/m2; 6) the same as 5 after 15-min incubation in darkness.
Thus, at the earliest stages of etiolated leaf greening, the active form should be identified with the previously unnoticed short-wavelength form having the fluorescence maximum at 638 nm (Pchlide 638).
Figure 9 displays spectral changes in fluorescence (a) and absorption (b) of etiolated leaves at a somewhat later stage of etiolation, when the main chlorophyll precursor form with the fluorescence peak at 655 nm becomes predominant. The comparison of absorption and fluorescence spectra indicates that the quantum yield of produced chlorophyllide forms undergoes changes both in the course of photoreaction and in the dark processes. The intense illumination is accompanied by the spectral shift of photoproducts toward longer wavelengths (cf. curves 3 and 5 in Fig. 9b). This shift suggests the involvement of Pchlide 655/650 form (in contrast to Pchlide 638, see above) in both sequential photoreactions at the terminal stage of chlorophyll biosynthesis.
On the whole, preliminary spectral studies of etiolated leaf fluorescence during native photochemical transformations at room temperature demonstrate the complexity of processes occurring in the leaf, as compared to the relatively simple operation scheme established for the model systems.
ROLE OF PROTOCHLOROPHYLLIDE AGGREGATION AND POR AGGREGATION IN
FORMATION OF PHOTOCHEMICALLY ACTIVE COMPLEX
Already in early studies [157], an assumption was put forward that the active protochlorophyllide form, Pchlide 655/650, is an aggregate of pigment molecules. The aggregated nature of long-wavelength forms is evident from some spectral features. First, the spectral maxima of these forms are shifted to longer wavelengths compared to spectra of pigment solutions. The absorption and fluorescence bands for the active Pchlide 655/650 form are narrower by a factor of 1.5-2.0 compared to those of Pchlide 633/628; this provides evidence for interactions between the electron structures of chromophores [200, 201]. After disaggregating treatments (moderate heating, addition of organic solvents or detergents), the long-wavelength spectral bands diminished or disappeared, while the short-wavelength bands turned more pronounced [12, 157, 202-205].
The analysis of spectral properties of protochlorophyll(ide) in model systems, compared to spectral properties in vivo, also supported the proposition that the active protochlorophyllide form is a pigment aggregate. Many researchers noted the close similarity (or identity) in spectral characteristics for the active protochlorophyllide form and for the aggregates of protochlorophyllous pigments in solutions [206-213]; the spectra of pigment aggregates in solutions characteristically display a bathochromic shift of absorption bands (including the Soret band). The coincident peak positions of the main Soret band for protochlorophyllide aggregates in vitro and of the band assigned to active protochlorophyllide form Pchlide 655/650 in vivo (around 460 nm) provides evidence for the aggregated state of Pchlide 655/650. The spectra of hard layers of protochlorophyllous pigments (treated with ammonia vapors) in the red region are also quite close to the spectra of chlorophyll precursor in vivo [214-220].
The energy interaction characteristic of pigment aggregates also applies to protochlorophyllide molecules in etiolated leaves. This conclusion is supported by studies of fluorescence polarization, circular dichroism [187-189], and excitation energy transfer [87, 165, 181-183, 221-225].
Thus, there is ample evidence that the photoactive forms of protochlorophyllide in vivo exist in an aggregated state.
Since one POR molecule reduces only one protochlorophyllide molecule in the catalytic site [34, 49], the active pigment-protein complex is thought to consist of several active ternary complexes. Moreover, the distances between pigment molecules should be sufficiently short to ensure excitation energy transfer by the inductive resonance mechanism. Hence, the POR enzyme should be capable of forming oligomers. The assumptions that POR in vivo exists in aggregated state appeared in earlier works too [227-229]. It should be noted that proteins attributed to the superfamily RED are characterized by the ability to form aggregates [37]. The major part of enzymes from this family operates in the form of dimers or tetramers. Investigation of POR in vitro established that the aggregation of the enzyme is beneficial for its enzymatic activity [226]. The fractionation of wheat PLB by isoelectric focusing at different stages of greening clarified that, even at the terminal stage of chlorophyll biosynthesis, during Shibata's shift, the pigment and POR are associated in the same fraction [230]. The authors supposed that the short-wavelength spectral shift at this stage is related to the destruction of POR aggregates, which results in disaggregation of the pigment. Using cross-binding technique, Wiktorsson et al. obtained evidence that prolamellar bodies contain POR in the aggregated state, represented mainly by dimers [230]. Martin et al. [226] applied gel filtration chromatography to study purified MBP-POR total protein (maltose-binding protein-POR), which was obtained by overexpression of pea POR in E. coli, and showed convincingly that the major part of native pigment exists in the dimeric state [226].
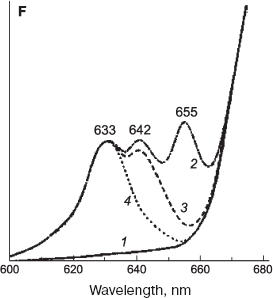
Chahdi et al. [231] examined photoactive POR complexes isolated from etiolated wheat leaves and purified with gel chromatography. A large molecular mass of these complexes (112 kD) together with spectral maxima of absorption at 640 nm and fluorescence at 643 nm suggest the aggregation of enzyme subunits. The use of comparatively low detergent concentrations for solubilization of prolamellar bodies yielded heavy particles with molecular mass of 1080 ± 250 kD whose spectral properties corresponded to the main active form of protochlorophyllide in vivo, Pchlide 655/650. In these complexes, POR was the dominant polypeptide. The gel chromatography of illuminated samples revealed rapid disaggregation of the complex after the photoconversion of protochlorophyllide.
According to some data [228, 231, 232], the protochlorophyllide Pchlide 643/638 is a dimer, while Pchlide 655/650 should be a larger aggregate. This view is supported by observations that the absorption and fluorescence bands are narrower for Pchlide 655/650 than for Pchlide 643/638.
Thus, the application of various experimental approaches yielded evidence that the active ternary complex Pchlide-NADPH-POR exists in the aggregated state in the form of dimers or of larger size oligomers. Lipids seem to promote the aggregation of pigment-protein complex (see below).
The nature of long-wavelength spectral forms is supposedly related to the occurrence of large aggregates of protochlorophyllide. This notion is substantiated by the presence of similar spectral bands for protochlorophyll aggregated in model systems [207, 208, 210, 215, 233, 234] and for protochlorophyll in seed coats of some plant species [235-238]. According to spectra of circular dichroism, the seed coats contain the long-wavelength protochlorophyll form in a crystalline form [237]. The formation of protochlorophyllide aggregates is promoted by aggregation of the photoenzyme POR, which facilitates the interaction of porphyrin rings of the pigment molecules.
ADDITIONAL COMPONENTS ASSOCIATED WITH ACTIVE PIGMENT-ENZYME
COMPLEX OF ETIOLATED LEAVES
In addition to the main components of the active pigment-enzyme complex of etioplasts (protochlorophyllide, NADPH, and photoenzyme POR), other components contribute to functioning of this complex in vivo.
Flavins as a component of active complex of chlorophyll biosynthesis. Data obtained with the use of optical spectroscopy [239] and preparative techniques [240] indicated that the photoactive complex comprises flavins whose function is yet unresolved. Walker and Griffiths [240] put forward a proposal about the flavin nature of the POR coenzyme. This view was based on several lines of evidence: (i) the POR activity was inhibited by quinacrine and trifluoroperazine known as flavoprotein antagonists; (ii) the preparations containing POR were able to reduce cytochrome c; (iii) in membranes enriched with POR content, FAD was coisolated together with the enzyme. Nayar et al. [241] examined photoinduced interactions of porphyrins and flavins in model systems. Based on the data obtained, they supposed that flavins in vivo could act as electron donors in the reaction of protochlorophyllide photoreduction. Later Griffiths revisited his own viewpoint on the presence of flavins in the active pigment-protein complex [242]. He succeeded in isolation of flavin-free POR that was able to catalyze photoreduction of protochlorophyllide. Since the artificial complex Pchlide-NADPH-POR was photoactive in the absence of flavins, Griffiths concluded that flavins are not constituents of the active complex in vivo. However, in our opinion, the retention of POR activity for the purified enzyme in vitro is insufficient evidence to conclude that flavins are not involved in chlorophyll biosynthesis in vivo, in whole etiolated leaves.
We investigated light-induced changes of fluorescence spectra in etiolated maize leaves at 77K for a wide spectral range including the fluorescence maxima for NADPH (470 nm) and flavins (525 nm) [239]. We observed that the light-induced fluorescence quenching of the active Pchlide form occurred concurrently with the decrease of flavin fluorescence band. This was apparently due to the photoreduction of flavin, because only oxidized flavin is the fluorescent form. After increasing the temperature, the accumulation of primary chlorophyllide forms was paralleled by the rise in flavin fluorescence (peak at 525 nm) to a level exceeding the initial fluorescence in darkened samples. This overshoot indicates the increase in concentration of oxidized flavins. The results of this work provide evidence for a possible involvement of flavins in the primary photochemical reactions of protochlorophyllide photoreduction. The photoreduction of chlorophyll precursor in vivo and in vitro might involve different mechanisms. In our view, the current knowledge is insufficient to fully exclude the role of flavins in organization and operation of the active pigment-protein complex of protochlorophyllide in vivo.
Role of lipids in formation of active complex of protochlorophyllide. Reinbothe et al. [243] investigated the influence of galacto- and sulfolipids extracted from barley etioplasts on the spectral properties of in vitro created artificial active complex composed of zinc protopheophorbide a (ZnPPhea), and zinc protopheophorbide b (ZnPPheb), enzymes PORA and PORB from barley, and NADPH. The fluorescence spectrum of the lipid-free complex had the main maximum at 630 nm. The addition of the mixture of galacto- and sulfolipids shifted the peak to 655 nm, the position characteristic of the active complex in vivo. Apparently, lipids promote the aggregation of pigment-protein complex. Klement et al. [244] reached the same conclusion from studying the influence of glycerol and chloroplast lipids on spectral changes of artificial pigment-enzyme complexes. Considering that monogalactosyl diacylglycerol and digalactosyl diacylglycerol are constituents of the lipid bilayer in prolamellar bodies [245], the authors of work [244] selected these plastid lipids for the addition to artificial complexes composed of POR, NADPH, and zinc protopheophorbide a. The addition of lipids shifted the long-wavelength absorption maximum of the complex from 628 to 646 nm. After illumination of the samples, the authors noted the production of pheophorbide with the absorption maximum at 678 nm; this maximum shifted in darkness to 668 nm. The authors believe that their data prove the involvement of these two lipid species in the formation of active long-wavelength form of the chlorophyll precursor in vitro and, possibly, in vivo.
Minor polypeptides as components of the chlorophyll precursor native complex. The protochlorophyllide in etioplasts is bound not only to POR but also to other polypeptides with molecular masses of 70, 41, 17, and 14 kD [246]. For elucidating the biogenesis pathways of photosynthetic apparatus, the finding was particularly important that etioplasts contain polypeptides identical to the components of PS2 reaction centers [247, 248]. The application of fast radioactive labeling method revealed that darkened etioplasts contain minor quantities of virtually all principal apoproteins for the reaction centers of both photosystems: P700, CP47, CP43, D2, and D1 [249-251]. However, the accumulation of these apoproteins commenced only upon illumination.
Based on numerous studies, one may conclude that the active complex at the terminal stage of chlorophyll biosynthesis includes the following principal components: active forms of protochlorophyllide (Pchlide 655/650 and Pchlide 643/637), the photoenzyme POR whose tyrosine group acts as a proton donor (for the position C17), NADPH that serves as second proton (hydride-ion) donor, and, possibly, flavins as electron donors or carriers. Furthermore, lipids play an important role in the formation of the active complex.
It is not excluded that some minor polypeptides different from POR, specifically protein constituents of the two photosystems of photosynthesis, are also associated with the active complexes of the chlorophyll precursor or, probably, are included in the composition of some minor active complexes. The HPLC analysis of photoactive Pchlide-POR complexes isolated from wheat plants gave grounds to infer a possible association of zeaxanthin and violaxanthine with the photoactive complex of protochlorophyllide [231].
During the last few decades, significant results have been obtained in studying the light stage of chlorophyll biosynthesis. The composition of the complex was elucidated where the photoreduction of dark-accumulated chlorophyll precursor occurs. The complex comprises three components: protochlorophyllide, NADPH as hydrogen atom donor, and the photoenzyme protochlorophyll oxidoreductase (POR). The main properties of the photoenzyme POR were determined, and the reaction of protochlorophyllide photoreduction was reconstituted in the model system, i.e. artificial ternary complex.
At the same time, elaborated spectral investigations dealt with accumulation and reduction of the chlorophyll precursor in intact plant cells and leaves have shown that the process occurring in the cell is much more complicated than in the model systems. The heterogeneity of chlorophyll precursor forms, the differential pathways of their transformation to chlorophyll, and the multistage arrangement of photochemical and dark reactions [178] indicate that the process results in production of several functionally active pigment-protein complexes required for the construction of photosynthetic apparatus. The results of investigations allow researchers to identify the pathways leading to pigment incorporation into two different photochemical systems of photosynthesis. Hopefully, the next stage of investigations will clarify the nature of heterogeneity for native forms of chlorophyll precursor, the structure of these forms, and their involvement in initiation of principal pigment-protein complexes of the photosynthetic machinery. For resolving the nature of active complexes of chlorophyll precursor, the study of these complexes in live functional cells becomes particularly significant.
This work was supported by the Russian Foundation for Basic Research, project No. 05-04-49377a.
REFERENCES
1.Fischer, H. (1940) Naturwissenschaften,
28, 401-405.
2.Ficher, H., and Oestreicher, A. (1940) Z.
Physiol. Chem., 262, 243.
3.Ficher, H., Mittenzwei, H., and Oestreicher, A.
(1939) Z. Physiol. Chem., 257, IV.
4.Belyaeva, O. B. (2003) Photosynth. Res.,
76, 405-411.
5.Lyubimenko, V. N. (1923) Izv. Ros. Akad.
Nauk, Ser. 6, 17, 129.
6.Lyubimenko, V. N., and Gyubbenet, E. K. (1930)
Izv. Estestv.-Nauch. Inst. Lesgafta, 16, 165.
7.Smith, J. H. C. (1948) Arch. Biochem.,
19, 449-454.
8.Smith, J. H. C. (1952) Year Book Carneg.
Inst., 51, 151.
9.Krasnovsky, A. A., and Kosobutskaya, L. M. (1952)
Dokl. Akad. Nauk SSSR, 85, 177-180.
10.Kosobutskaya, L. M., and Krasnovsky, A. A. (1954)
Biokhimiya, 19, 37-44.
11.Smith, J. H. C., and Benitez, A. (1953)
Carnegie Inst. Wash. Year Book, 52, 151-153.
12.Smith, J. H. C., and Benitez, A. (1954) Plant
Physiol., 29, 135-143.
13.Smith, J. H. C., and Kupke, D. W. (1956)
Nature, 178, 751-752.
14.Smith, J. H. C., Kupke, D. W., and Giese, A. T.
(1956) Carn. Inst. Wash. Yearly Book, 55, 243-245.
15.Smith, J. H. C. (1960) in Comparative
Biochemistry of Photoreactive Systems (Allen, M. B., ed.) Academic
Press, New York, pp. 257-277.
16.Smith, J. H. C. (1961) in Biological Structure
and Function, Vol. 2 (Goodwin, T. W., and Linberg, O., eds.)
Academic Press, London, pp. 325-338.
17.Boardman, N. K. (1962) Biochim. Biophys.
Acta, 62, 63-79.
18.Wolf, J. B., and Price, L. (1956) Plant
Physiol., 31, 31.
19.Godnev, T. N., Shlyk, A. A., and Lyakhnovich, Ya.
P. (1957) Fiziol. Rast., 4, 393-400.
20.Griffiths, W. T. (1974) FEBS Lett.,
46, 301-304.
21.Griffiths, W. T. (1975) Biochem. J.,
152, 623-635.
22.Griffiths, W. T., and Mapleston, R. E. (1978) in
Chloroplast Development (Akoyunoglou, G., and
Argyroudi-Akoyunoglou, J. H., eds.) Amsterdam, pp. 99-104.
23.Griffiths, W. T. (1978) Biochem. J.,
174, 681-692.
24.Suzuki, J. Y., and Bauer, C. E. (1995) Proc.
Natl. Acad. Sci. USA, 92, 3749-3753.
25.Fujita, Y. (1996) Plant. Cell. Physiol.,
37, 411-421.
26.Armstrong, G. A. (1998) J. Photochem.
Photobiol. B, 43, 87-100.
27.Oliver, R. P., and Griffiths, W. T. (1980)
Biochem. J., 191, 277-280.
28.Apel, K., Santel, H. J., Redlinger, T. E., and
Falk, H. (1980) Eur. J. Biochem., 111, 251-258.
29.Oliver, R. P., and Griffiths, W. T. (1981)
Biochem. J., 195, 93-101.
30.Apel, K. (1981) Eur. J. Biochem.,
120, 89-93.
31.Teakle, R., and Griffiths, W. T. (1993)
Biochem. J., 296, 225-230.
32.Reinbothe, S., Reinbothe, C., Lebedev, N., and
Apel, K. (1996) Plant Cell, 8, 763-769.
33.Baker, M. E. (1994) Biochem. J.,
300, 605-607.
34.Wilks, H. M., and Timko, M. P. (1995) Proc.
Natl. Acad. Sci. USA, 92, 724-728.
35.Birve, S., Selstam, E., and Johansson, L. (1996)
Biochem. J., 317, 549-555.
36.Labesse, G., Vidal-Cros, A., Chomilie, J.,
Gaudry, M., and Morno, J. P. (1994) Biochem. J., 304,
95-99.
37.Jornvall, H., Persson, B, Krook, M., Atrian, S.,
Gonzalez-Duarte, R., Jeffery, J., and Ghosh, D. (1995)
Biochemistry, 34, 6003-6013.
38.Tanaka, N., Nonaka, T., Tanabe, T., Yoshimoto,
T., Tsuru, D., and Mitsui, Y. (1996) Biochemistry, 35,
7715-7730.
39.Tanaka, N., Nonaka, T., Nakanishi, M.,
Deyashiki, Y., Hara, A., and Mitsui, Y. (1996) Structure,
4, 33-45.
40.Townley, H. E., Sessions, R. B., Clarke, A. R.,
Dafforn, T. R., and Griffiths, W. T. (2001) Proteins, 44,
329-335.
41.Schultz, R., Steinmuller, K., Klaas, M.,
Forreiter, C., Rasmussen, S., Hiller, C., and Apel, A. (1989)
Genetics, 217, 355-361.
42.Oliver, R. P., and Griffiths, W. T. (1982)
Plant Physiol., 70, 1019-1025.
43.Griffiths, W. T. (1980) Biochem. J.,
186, 267-278.
44.Dahlin, C., Aronsson, H., Wilks, H. M., Lebedev,
N., Sundqvist, C., and Timko, M. (1999) Plant Mol. Biol.,
39, 309-323.
45.Wiktorsson, B., Engdahl, S., Zhong, L. B., Boddi,
B., Ryberg, M., and Sundqvist, C. (1993) Photosynthetica,
29, 205-218.
46.Martin, G. E. M., Timko, M. P., and Wilks, H. M.
(1997) Biochem. J., 325, 139-145.
47.Griffiths, W. T., McHugh, T., and Blankenship, R.
E. (1996) FEBS Lett., 398, 235-238.
48.Lebedev, N., and Timko, M. (1999) Proc. Natl.
Acad. Sci. USA, 96, 17954-17959.
49.Klement, H., Helfrich, M., Oster, U., Schoch, S.,
and Rudiger, W. (1999) Eur. J. Biochem., 265,
862-874.
50.Heyes, D. J., and Hunter, C. N. (2002)
Biochem. Soc. Trans., 30, 601-604.
51.Lebedev, N., Karginova, O., McIvor, W., and
Timko, M. (2001) Biochemistry, 40, 12562-12574.
52.Schoefs, B., and Franck, F. (2003) Photochem.
Photobiol., 78, 543-557.
53.Horton, P., and Leech, R. M. (1975) Plant
Physiol., 56, 113-120.
54.Schoch, S., Helfrich, M., Wiktorsson, B.,
Sundqvist, C., Rudiger, W., and Ryberg, M. (1995) Eur. J.
Biochem., 229, 291-298.
55.Griffiths, W. T. (1991) in Chlorophyll
(Sheer, H., ed.) CRC Press, Boca Raton, FL, pp. 433-450.
56.Kotzabasis, K., Schuring, M. P., and Senger, H.
(1989) Physiol. Plant., 75, 221-226.
57.Ignatov, N. V., and Litvin, F. F. (1995)
Biochemistry (Moscow), 60, 1429-1438.
58.Ignatov, N. V., and Litvin, F. F. (1996)
Photosynth. Res., 50, 271-283.
59.Bjorn, L. O. (1963) Physiol. Plant.,
16, 142-150.
60.Bjorn, L. O. (1969) Physiol. Plant.,
22, 1-17.
61.Rebeiz, C. A., Yaghi, M., Abou-Haidar, M., and
Castelfranco, P. A. (1970) Plant Physiol., 46, 57-63.
62.Lang, F., Vorobyeva, L. M., and Krasnovsky, A. A.
(1971) Mol. Biol. (Moscow), 5, 366-374.
63.Cohen, C. E., and Schiff, J. A. (1976)
Photochem. Photobiol., 24, 555-566.
64.Belanger, F. C., and Rebeiz, C. A. (1980) J.
Biol. Chem., 255, 1266-1272.
65.Belanger, F. C., and Rebeiz, C. A. (1980)
Biochemistry, 19, 4875-4883.
66.Belanger, F. C., and Rebeiz, C. A. (1980)
Plant Sci. Lett., 18, 343-350.
67.Chereskin, B. M., Wong, Y. S., and Castelfranco,
P. A. (1982) Plant Physiol., 70, 987-993.
68.Belanger, F. C., and Rebeiz, C. A. (1985) J.
Biol. Chem., 255, 1266-1272.
69.Whyte, B. J., and Griffiths, W. T. (1993)
Biochem. J., 291, 939-944.
70.Adra, A. N., and Rebeiz, C. A. (1998)
Photochem. Photobiol., 68, 852-856.
71.Bombart, P., and Dujardin, E. (1984) in
Protochlorophyllide Reduction and Greening (Sironval, C., and
Brouers, M., eds.) Martinus Nijhoff/Dr. Junk Publishers, The Hague, pp.
175-179.
72.Krasnovsky, A. A., Bistrova, M. I., and
Safronova, I. A. (1984) in Protochlorophyllide Reduction and
Greening (Sironval, C., and Brouers, M., eds.) Martinus Nijhoff/Dr.
Junk Publishers, The Hague, pp. 331-339.
73.Helfrich, M., Schoch, S., Schafer, W., Ryberg,
M., and Rudiger, W. (1996) J. Am. Chem. Soc., 118,
2606-2611.
74.Nakamura, A., and Watanabe, T. (1998) FEBS
Lett., 426, 201-204.
75.Ikeuchi, M., and Murakami, S. (1982) Plant
Cell Physiol., 23, 575-583.
76.Ikeuchi, M., and Murakami, S. (1982) Plant
Cell Physiol., 23, 1089-1099.
77.Ryberg, M., and Sundqvist, C. (1982) Physiol.
Plant., 56, 125-132.
78.Selstam, E. A., Widell, A., and Johansson, L. B.
(1987) Physiol. Plant., 70, 209-214.
79. Benli, M., Schulz, R., and Apel, K. (1991) Plant Mol.
Biol., 16, 615-625.
80.Dehesh, K., Klaas, M., Hauser, I., and Apel, K.
(1986) Planta, 169, 162-171.
81.Armstrong, G., Runge, S., Frick, G., Sperling,
U., and Apel, K. (1995) Plant Physiol., 108,
1505-1517.
82.Holtorf, H., Reinbothe, S., Reinbothe, C.,
Bereza, B., and Apel, K. (1995) Proc. Natl. Acad. Sci. USA,
92, 3254-3258.
83.Frick, G., Su, Q., Apel, K., and Armstrong, G. A.
(2003) Plant J., 35, 141-153.
84.Sperling, U., van Cleve, B., Frick, G., Apel, K.,
and Armstrong, G. A. (1997) Plant J., 12, 649-658.
85.Sperling, U., Franck, F., van Cleve, B., Frick,
G., Apel, K., and Armstrong, G. A. (1998) Plant Cell, 10,
283-296.
86.Sperling, U., Frick, G., van Cleve, B., and Apel,
K. (1999) in The Chloroplast: From Molecular Biology to
Biotechnology (Argyroudi-Akoyunoglou, J. H., and Senger, H., eds.)
Kluwer, Dordrecht, pp. 97-102.
87.Franck, F., Sperling, U., Frick, G., Pochert, B.,
van Cleve, B., Apel, K., and Armstrong, G. A. (2000) Plant
Physiol., 124, 1678-1696.
88.Santel, H.-J., and Apel, K. (1981) Eur. J.
Biochem., 120, 95-103.
89.Reinbothe, C., Apel, K., and Reinbothe, S. (1995)
Mol. Cell. Biol., 15, 6206-6212.
90.Reinbothe, S., Reinbothe, C., Apel, K., and
Lebedev, N. (1996) Cell, 86, 703-705.
91.Schunmann, P. H., and Ougham, H. J. (1996)
Plant. Mol. Biol., 31, 529-537.
92.Skinner, J. S., and Timko, M. P. (1998) Plant
Cell Physiol., 39, 795-806.
93.Kaneko, T., Sato, S., Kotani, H., Tanaka, A.,
Asamizu, E., Nakamura, Y., Miyajima, N., Hirosawa, M., Sugiura, M.,
Sasamoto, S., Kimura, T., Hosouchi, T., Matsuno, A., Muraki, A.,
Nakazaki, N., Naruo, K., Okamura, S., Shimpo, S., Takeuchi, C., Wada,
T., Watanabe, A., Yamada, M., Ysaduda, M., and Tabata, S. (1996) DNA
Res., 3, 109-136.
94.Heyes, D. J., Martin, G. E. M., Reid, R. T.,
Hunter, C. N., and Wilks, H. M. (2000) FEBS Lett., 483,
47-51.
95.Li, J., and Timko, M. P. (1996) Plant Mol.
Biol., 30, 15-37.
96. Kuroda, H., Masuda, T., Fusada, N., Ohta, H., and Takamiya,
K. (2000) Plant Cell Physiol., 41, 226-229.
97.Spano, A. J., He, Z., Michel, H., Hunt, D. F.,
and Timko, M. P. (1992) Plant Mol. Biol., 18,
967-972.
98.Oosawa, N., Masuda, T., Awai, K., Fusada, N.,
Shimada, H., Ohta, H., and Takamiya, K. (2000) FEBS Lett.,
474, 133-136.
99.Su, Q., Frick, G., Armstrong, G., and Apel, K.
(2001) Plant Mol. Biol., 47, 805-813.
100. Kuroda, H., Masuda, T., Fusada, N., Ohta, H., and Takamiya,
K. (1995) Plant Cell Physiol., 41, 226-229.
101.Takio, S., Nakao, N., Suzuki, T., Tanaka, K.,
Yamamoto, I., and Satoh, T. (1998) Plant Cell Physiol.,
39, 665-669.
102.Masuda, T., Fusada, N., Oosawa, N., Takamatsu,
K., Yamamoto, Y. Y., Ohta, M., Nakamura, K., Goto, K., Shibata, D.,
Shirano, Y., Hayashi, H., Kato, T., Tabata, S., Shimada, H., Ohta, H.,
and Takamiya, K. (2003) Plant Cell Physiol., 44,
963-974.
103.He, Z. H., Li, J., Sundqvist, C., and Timko, M.
P. (1994) Plant Physiol., 106, 537-546.
104.Yoshida, K., Chen, R. M., Tanaka, A., Teramoto,
H., Tanaka, R., Timko, M. P., and Tsuji, H. (1995) Plant
Physiol., 109, 231-238.
105.Boardman, N. K., and Anderson, J. M. (1964)
Aust. J. Biol. Sci., 17, 86-92.
106.Leyon, H. (1953) Exp. Cell. Res.,
5, 520-529.
107.Von Wettstein, D. (1959) in Developmental
Cytology (Rudnick, D., ed.) Ronald Press, New York, pp.
123-160.
108.Ryberg, M., and Sundqvist, C. (1982)
Physiol. Plant., 56, 125-132.
109.Ryberg, M., and Sundqvist, C. (1982)
Physiol. Plant., 56, 133-138.
110.Lindsten, A., Ryberg, M., and Sundqvist, C.
(1988) Physiol. Plant., 72, 167-176.
111.Shaw, P. J., Henwood, J. A., Oliver, R. P., and
Griffiths, W. T. (1985) Eur. J. Cell Biol., 39,
50-55.
112.Ryberg, M., and Dehesh, K. (1986) Physiol.
Plant., 66, 616-624.
113.Klein, S., and Schiff, J. A. (1972) Plant
Physiol., 49, 619-626.
114.Rowe, J. D., and Griffiths, W. T. (1981)
Biochem. J., 311, 417-424.
115.Schultz, R., Steinmuller, K., Klaas, M.,
Forreiter, C., Rasmussen, S., Hiller, C., and Apel, K. (1989) Mol.
Gen. Genet., 217, 355-361.
116.Darrah, P. M., Kay, S. A., Teakle, G. R., and
Griffiths, W. T. (1990) Biochem. J., 265, 789-798.
117. Joyard, J., Block, M., Pineau, B., Albrieux, C., and Douce,
R. (1990) J. Biol. Chem., 265, 21820-21827.
118.Pineau, B., Gerard-Hirne, C., Douce, R., and
Joyard, J. (1993) Plant Physiol., 102, 821-828.
119.Dahlin, C., Sundqvist, C., and Timko, M. P.
(1995) Plant Mol Biol., 29, 317-330.
120.Masuda, T., and Takamiya, K. (2004)
Photosynth. Res., 81, 1-29.
121.Savchenko, G. E., Abramchik, L. M.,
Serdyuchenko, E. V., and Chaika, M. T. (1989) Dokl. Akad. Nauk Bel.
SSR, 33, 660-663.
122.Pineau, B., Dubertret, G., Joyard, J., and
Douce, R. (1986) J. Biol. Chem., 261, 9210-9215.
123.Aronsson, H., Sundqvist, C., Timko, M. P., and
Dahlin, C. (2001) FEBS Lett., 502, 11-15.
124.Aronsson, H., Sundqvist, C., and Dahlin, C.
(2003) Plant Mol. Biol., 51, 1-7.
125.Dahlin, C., Aronsson, H., Wilks, H. M.,
Lebedev, N., Sundqvist, C., and Timko, M. (1999) Plant Mol.
Biol., 39, 309-323.
126.Godnev, T. N., Galaktionov, S. G., and Raskin,
V. I. (1968) Dokl. Akad. Nauk SSSR, 181, 237-240.
127.Bjorn, L. O. (1963) Physiol. Plant.,
16, 142-150.
128.Boardman, N. K. (1966) in The
Chlorophylls (Vernon, L. P., and Seely, G. R., eds.) Academic
Press, New York, pp. 437-479.
129.Lang, F., Vorobyeva, L. M., and Krasnovsky, A.
A. (1971) Mol. Biol. (Moscow), 5, 366-374.
130.Rebeiz, C. A., and Castelfranco, P. A. (1973)
Ann. Rev. Plant Physiol., 24, 129-172.
131.Rebeiz, C. A., Smith, B. B., Mattheis, J. R.,
Cohen, C. E., and McCarthy, S. A. (1978) in Chloroplast
Development (Akoyounoglou, G., and Argyroudi-Akoyounoglou, J. H.,
eds.) Amsterdam, pp. 59-76.
132.Boddi, B., Lindsten, A., Ryberg, M., and
Sundqvist, C. (1989) Physiol. Plant., 76, 135-143.
133.Ellsworth, R. K., and Nowak, C. (1974) Ann.
Biochem., 57, 534-546.
134.Liljenberg, C. L. (1974) Physiol.
Plant., 32, 208-213.
135.Liljenberg, C. L. (1977) in Lipids and Lipid
Polymers in Higher Plants (Tevini, M., and Lichtentaler, H. K.,
eds.) Berlin, pp. 259-270.
136.Shioi, Y., and Sasa, T. (1982) Plant Cell
Physiol., 23, 1315-1321.
137.Shioi, Y., and Sasa, T. (1983) Plant Cell
Physiol., 24, 835-840.
138.Aronoff, S., and Ellsworth, R. K. (1968)
Photosynthetica, 2, 288-297.
139.Jones, O. T. G. (1979) in The Porphyrins
(Dolphyn, D., ed.) Vol. 6, New York, pp. 179-232.
140.Belanger, F. C., and Rebeiz, C. A. (1979)
Biochem. Biophys. Res. Commun., 88, 365-372.
141.Belanger, F. C., Duggan, J. X., and Rebeiz, C.
A. (1982) J. Biol. Chem., 257, 4849-4858.
142.Wu, S. M., Mayasich, M., and Rebeiz, C. A.
(1989) Analyt. Biochem., 178, 294-300.
143.Carey, E. E., and Rebeiz, C. A. (1985) Plant
Physiol., 79, 1-6.
144.Carey, E. E., Tripathy, B. C., and Rebeiz, C.
A. (1985) Plant Physiol., 79, 1059-1063.
145.Granick, S., and Gassman, M. L. (1970) Plant
Physiol., 45, 201-205.
146.Rebeiz, C. A., Belanger, F. C., McCarthy, S.
A., Fressinet, G., Duggan, J. X., Wu, S. M., and Mattheis, J. R. (1981)
Proc. 5th Int. Congr. Photosynth. (Akoyunoglou, G., ed.) Vol. 5,
Int. Sci. Services, Jerusalem, pp. 197-212.
147.Duggan, J. X., and Rebeiz, C. A. (1982)
Plant Sci. Lett., 24, 27-37.
148.Duggan, J. X., and Rebeiz, C. A. (1982)
Plant Sci. Lett., 27, 137-145.
149.Rebeiz, C. A., and Lascelles, J. (1982) in
Photosynthesis (Govindjee, ed.) New York, pp. 699-780.
150.Rebeiz, C. A., Wu, S. M., Kuhadja, M., Daniell,
H., and Perkins, E. J. (1983) Mol. Cell. Biochem., 57,
97-125.
151.Koski, V. M., and Smith, J. H. C. (1948) J.
Amer. Chem. Soc., 70, 3558-3562.
152.Krasnovsky, A. A., and Voinovskaya, K. K.
(1949) Dokl. Akad. Nauk SSSR, 66, 663-666.
153.Monteverde, N. A., and Lubimenko, W. N. (1911)
Biologisches Centralblat, 31, 481-498.
154.Koski, V. M., French, C. S., and Smith, J. H.
C. (1951) Arch. Biochem. Biophys., 31, 1-17.
155.Shibata, K. (1956) Carn. Inst. Wash. Yearly
Book, 1956, 55, 248-250.
156.Shibata, K. (1957) J. Biochem. (Tokyo),
44, 147-173.
157.Litvin, F. F., and Krasnovsky, A. A. (1957)
Dokl. Akad. Nauk SSSR, 117, 106-109.
158.Litvin, F. F., and Krasnovsky, A. A. (1958)
Dokl. Akad. Nauk SSSR, 120, 764-767.
159.Litvin, F. F., and Krasnovsky, A. A. (1959)
Izv. Akad. Nauk SSSR, 120, 764-767.
160.Lang, F., Vorobyeva, L. M., and Krasnovsky, A.
A. (1968) Dokl. Akad. Nauk SSSR, 183, 711-714.
161.Lang, F., Vorobyeva, L. M., and Krasnovsky, A.
A. (1969) Biokhimiya, 34, 257-265.
162.Lang, F., Vorobyeva, L. M., and Krasnovsky, A.
A. (1969) Biofizika, 14, 245-255.
163.Lang, F., Vorobyeva, L. M., and Krasnovsky, A.
A. (1971) Mol. Biol. (Moscow), 5, 366-374.
164.Sironval, C., Brouers, M., Michel, J. M., and
Kuyper, Y. (1968) Photosynthetica, 2, 268-287.
165.Kahn, A., Boardman, N. K., and Thorne, S. W.
(1970) J. Mol. Biol., 48, 85-101.
166.Litvin, F. F., and Stadnichuk, I. N. (1980)
Fiziol. Rast., 27, 1024-1032.
167.Boddi, B., Ryberg, M., and Sundqvist, C. (1992)
J. Photochem. Photobiol., 12, 389-401.
168.Boddi, B., and Franck, F. (1997) J.
Photochem. Photobiol. B: Biol., 41, 73-82.
169.Schoefs, B., Bertrand, M., and Franck, F.
(2000) Photochem. Photobiol., 72, 85-93.
170.Stadnichuk, I. N., Amirjani, M. R., and
Sundqvist, C. (2005) Photochem. Photobiol. Sci., 4,
230-238.
171.Bovey, F., Ogawa, T., and Shibata, K. (1974)
Plant Cell Physiol., 15, 1133-1137.
172.Cohen, C. E., and Rebeiz, C. A. (1981) Plant
Physiol., 67, 98-103.
173.Shioi, Y., and Sasa, T. (1984) Plant Cell
Physiol., 25, 131-137.
174.Franck, F., and Strazlka, K. (1992) FEBS
Lett., 309, 73-77.
175.Virgin, H. I. (1993) Physiol. Plant.,
89, 761-766.
176.Valter, G., Belyaeva, O. B., Ignatov, N. V.,
Krasnovsky, A. A., and Litvin, F. F. (1982) Biol. Nauki, No. 9,
35-39.
177.Sundqvist, C., and Dahlin, C. (1997)
Physiol. Plant., 100, 748-759.
178.Litvin, F. F., Belyaeva, O. B., and Ignatov, N.
V. (2000) Usp. Biol. Khim., 40, 3-42.
179.Akulovich, N. K. (1983) Formation and State
of Photoactive Protochlorophyllous Pigment [in Russian], Nauka i
Tekhnika, Minsk.
180.Mysliwa-Kurdziel, B., Amirjani, M. R.,
Strzalka, K., and Sundqvist, C. (2003) Photochem. Photobiol.,
78, 205-212.
181.Litvin, F. F., Efimtsev, E. I., and Ignatov, N.
V. (1976) Biofizika, 21, 307-312.
182.Ignatov, N. V. (1977) Investigation of
Photochemical Stage of Chlorophyll Biosynthesis: Author's abstract
of Candidate's dissertation [in Russian], Moscow.
183.Ignatov, N. V., and Litvin, F. F. (1981)
Biofizika, 26, 664-668.
184.Akulovich, N. K., and Raskin, V. I. (1971) in
Topics in Chlorophyll Biosynthesis [in Russian], Nauka i
Tekhnika, Minsk, pp. 5-52.
185.El Hamouri, B., and Sironval, C. (1979) FEBS
Lett., 103, 345-347.
186.Houssier, C., and Sauer, K. (1970) J. Amer.
Chem. Soc., 92, 779-791.
187.Schultz, A., and Sauer, K. (1972) Biochim.
Biophys. Acta, 267, 320-340.
188.Vaugan, G. D., and Sauer, K. (1974) Biochim.
Biophys. Acta, 347, 383-394.
189.El Hamouri, B., and Sironval, C. (1980)
Photobiochem. Photobiophys., 1, 219-223.
190.El Hamouri, B., Brouers, M., and Sironval, C.
(1981) Plant Sci. Lett., 21, 375-379.
191.Franck, F., Bereza, B., and Boddi, B. (1999)
Photosynth. Res., 59, 53-61.
192.Belyaeva, O. B., and Litvin, F. F. (1980)
Biofizika, 25, 617-623.
193.Belyaeva, O. B., and Litvin, F. F. (1981)
Photosynthetica, 15, 210-215.
194.Ignatov, N. V., and Litvin, F. F. (2001)
Biochemistry (Moscow), 66, 195-204.
195.Ignatov, N. V., and Litvin, F. F. (2002)
Photosynth. Res., 71, 195-207.
196.Franck, F., Berthelemy, X., and Strazlka, K.
(1993) Photosynthetica, 29, 185-194.
197.Litvin, F. F., Rikhireva, G. T., and
Krasnovskii, A. A. (1962) Biofizika, 7, 578-591.
198.Ignatov, N. V., and Litvin, F. F. (2002)
Biochemistry (Moscow), 67, 949-955.
199.Schoefs, B., and Franck, F. (1998) Plant
Physiol., 118, 1159-1168.
200.Kasha, M. (1963) Radiat. Res.,
20, 55-70.
201.Litvin, F. F., and Sineshchekov, V. A. (1965)
in Molecular Biophysics [in Russian], Nauka, Moscow, pp.
191-203.
202.Krasnovsky, A. A., Bystrova, M. I., and
Sorokina, A. V. (1961) Dokl. Akad Nauk SSSR, 136,
1227-1230.
203.Vorobyeva, L. M., and Krasnovsky, A. A. (1966)
Biokhimiya, 31, 578-584.
204.Butler, W. L., and Briggs, W. R. (1966)
Biochim. Biophys. Acta, 112, 45-53.
205.Dujardin, E., and Sironval, C. (1970)
Photosynthetica, 4, 129-138.
206.Seliskar, C., and Ke, B. (1968) Biochim.
Biophys. Acta, 153, 685-691.
207.Brouers, M. (1972) Photosynthetica,
6, 415-423.
208.Brouers, M. (1979) Photosynthetica,
13, 9-14.
209.Losev, A. P., and Gurinovich, G. P. (1969)
Biofizika, 14, 110-118.
210.Zenkevich, E. I., and Losev, A. P. (1976)
Mol. Biol. (Moscow), 10, 294-304.
211.Zenkevich, E. I., Kochubeev, G. A., Losev, A.
P., and Gurinovich, G. P. (1978) Mol. Biol. (Moscow), 12,
1002-1011.
212.Shlyk, A. A., Fradkin, L. I., and Kalinina, L.
M. (1973) in Topics in Biophotochemistry [in Russian], Nauka,
Moscow, pp. 122-132.
213.Kotzabasis, K., Senge, M., Seyfried, B.,
and Senger, H. (1990) Photochem. Photobiol., 52,
95-101.
214.Bystrova, M. I., and Krasnovsky, A. A. (1967)
Mol. Biol. (Moscow), 1, 362-272.
215.Bystrova, M. I., Lang, F., and Krasnovsky, A.
A. (1972) Mol. Biol. (Moscow), 6, 77-86.
216.Bystrova, M. I., Safronova, I. A., and
Krasnovsky, A. A. (1982) Mol. Biol. (Moscow), 16,
291-301.
217.Bystrova, M. I., Safronova, I. A., and
Krasnovsky, A. A. (1985) Mol. Biol. (Moscow), 19,
915-925.
218.Krasnovsky, A. A., and Bystrova, M. I. (1974)
in Chlorophyll (Shlyk, A. A., ed.) [in Russian], Nauka i
Tekhnika, Minsk, pp. 139-153.
219.Krasnovsky, A. A., Bystrova, M. I., and Lang,
F. (1971) Dokl. Akad. Nauk SSSR, 201, 1485-1488.
220.Canaani, O. D., and Sauer, K. (1977) Plant
Physiol., 60, 422-429.
221.Sironval, C. (1972) Photosynthetica,
6, 375-380.
222.Fradkin, L. I., and Shlyk, A. A. (1978) Zh.
Prikl. Spektrosk., 29, 1029-1039.
223.Henningsen, K. W., Thorne, S. W., and Bordman,
N. K. (1974) Plant Physiol., 53, 419-425.
224.Brouers, M., and Sironval, C. (1974)
Plant Sci. Lett., 2, 67-72.
225.Brouers, M. (1975) Photosynthetica,
9, 304-310.
226.Martin, G. E. M., Timko, M. P., and Wilks, H.
M. (1997) Biochem. J., 325, 139-145.
227.Belyaeva, O. B., and Litvin, F. F. (1989)
Photobiosynthesis of Chlorophyll [in Russian], MGU Publishers,
Moscow.
228.Boddi, B., Lindsten, A., Ryberg, M., and
Sundqvist, C. (1989) Physiol. Plant., 76, 135-143.
229.Boddi, B., Lindsten, A., Ryberg, M., and
Sundqvist, C. (1990) Photochem. Photobiol., 52,
83-87.
230.Wiktorsson, B., Ryberg, M., Gough, S., and
Sundqvist, C. (1992) Physiol. Plant., 85, 659-669.
231.Chahdi, M. A., Schoefs, B., and Franck, F.
(1998) Planta, 206, 673-680.
232.Wiktorsson, B., Engdahl, S., Zhong, L. B.,
Boddi, B., Ryberg, M., and Sundqvist, C. (1993) Photosynthetica,
29, 205-218.
233.Boddi, B., Soos, J., and Lang, F. (1980)
Biochim. Biophys. Acta, 593, 158-165.
234.Boddi, B., Kovacs, K., and Lang, F. (1983)
Biochim. Biophys. Acta, 722, 320-326.
235.Inada, Y., and Shibata, K. (1960) Plant Cell
Physiol., 1, 311-316.
236.Sundqvist, C., and Ryberg, H. (1979)
Physiol. Plant., 47, 124-128.
237.Sundqvist, C., Ryberg, H., Boddi, B., and Lang,
F. (1980) Physiol. Plant., 48, 297-301.
238.Ignatov, N. V., Belyaeva, O. B., Timofeev, K.
N., and Litvin, F. F. (1988) Biofizika, 33, 500-505.
239.Ignatov, N. V., Belyaeva, O. B., and Litvin, F.
F. (1993) Photosynthetica, 29, 235-241.
240.Walker, C. J., and Griffiths, W. T. (1988)
FEBS Lett., 239, 259-262.
241.Nayar, P., Brun, A., Harriman, A., and Begley,
T. P. (1992) J. Chem. Soc. Chem. Commun., 395-397.
242.Townley, H. E., Griffiths, W. T., and Nugent,
J. P. (1998) FEBS Lett., 422, 19-22.
243.Reinbothe, C., Lebedev, N., and Reinbothe, S.
(1999) Nature (London), 397, 80-84.
244.Klement, H., Oster, U., and Rudiger, W. (2000)
FEBS Lett., 480, 306-310.
245.Selstam, E., and Widell-Wigge, A. (1993) in
Pigment-Protein Complexes in Plastids: Synthesis and Assembly
(Sundqvist, C., and Ryberg, M., eds.) Academic Press, San Diego, pp.
241-277.
246.Redlinger, T. E., and Apel, K. (1980) Arch.
Biochem. Biophys., 200, 253-260.
247.Mullet, J. E., Klein, P. G., and Klein, R. R.
(1990) Proc. Natl. Acad. Sci. USA, 87, 4038-4042.
248.Klein, R. R., Camble, P. E., and Mullet, J. E.
(1988) Plant Physiol., 88, 1246-1256.
249.Eichacker, L. F., Paulsen, H., and Rudiger, W.
(1992) Eur. J. Biochem., 205, 17-24.
250.Eichacker, L. F., Muller, B., and Helfrich, M.
(1996) FEBS Lett., 395, 251-256.
251.Kim, J., Klein, P., and Mullet, J. (1994) J.
Biol. Chem., 269, 17918-17923.