Pineal Gland as an Endocrine Gravitational Lunasensor: Manifestation of Moon-Phase Dependent Morphological Changes in Mice
A. V. Gerasimov1, V. P. Kostyuchenko1, A. S. Solovieva2, and A. M. Olovnikov2*
1Siberian State Medical University, Moscovskii Trakt 2, 634050 Tomsk, Russia2Institute of Biochemical Physics, Russian Academy of Sciences, ul. Kosygina 4, 119334 Moscow, Russia; E-mail: olovnikov@gmail.com
* To whom correspondence should be addressed.
Received May 21, 2014
We found that some morphological properties of the pineal gland and submandibular salivary gland of mice are significantly distinct at the new and full moon. We suppose that the differences are initiated by the displacements of the electron-dense concretions in the secretory vesicles of pinealocytes. This presumably occurs under the influence of the gravitational field, which periodically changes during different phases of the moon. It seems that the pinealocyte is both an endocrine and gravisensory cell. A periodic secretion of the pineal gland probably stimulates, in a lunaphasic mode, the neuroendocrine system that, in turn, periodically exerts influence on different organs of the body. The observed effect probably serves, within the lifelong clock of a brain, to control development and aging in time.
KEY WORDS: pinealocytes, electron-dense concretions in secretory vesicles, lunaphasic changes of cell morphologyDOI: 10.1134/S0006297914100083
It has been known for 400 years that the pineal gland contains so-called “brain sand”. It is composed of calcified inclusions. Our experimental observations reported here are consistent with the hypothesis that argues that these inclusions are used in the brain as a component of a special lifelong clock that is necessary to control the course of development and then aging in time. The pineal gland, as known, is involved in processes that morphologically resemble neurosecretion. Melatonin is the best-known hormone of this gland, although its cells, pinealocytes, also contain other factors that could be involved in regulation of the body. Granular secretory vesicles are typical of the pineal gland; they are described in mice, rats, gerbils, voles, Djungarian hamsters, and cotton rats [1-7]. In pigs, the membranous bodies rich in the lamellar component [8] are also likely to be associated with the secretory process in pinealocytes. Earlier, it was predicted that cells of the pineal gland could respond to periodic changes in the gravitational field of the moon by a periodic secretion of their hormonal factors due to the gravity-dependent mechanical displacements of mineral inclusions within this endocrine gland [9, 10]. The pineal gland could thereby be a peculiar gravisensor, or in other words, a lunasensor. Owing to this, it could serve as a pacemaker responsible for the operation of a lifelong clock in many animal species, including humans. This lifelong clock could control the successive changes in the biological age of the body. Operation of such clock is needed to ensure the timely onset of different developmental processes. However, the lifelong clock persists ticking even after physiological maturation of the body has been completed. And just this could be the pivotal cause that gradually leads the organism to aging [9, 10]. According to this hypothesis, pinealocytes should change their secretory activity in the new and full moon, periodically modifying some of their own morphological features and other properties of the body as well, by exposing them to periodic changes in the profile of the neuroendocrine activity and secretion. Moon phase-associated changes in the profile of concentrations of melatonin have recently been described in biological fluid [11]. In the present study, we searched for lunar phase-dependent, i.e. lunaphasic, morphological changes both in the pineal gland and in the submandibular salivary gland. The salivary gland has granular departments that occupy almost half of the gland’s volume; it is known that they are able to respond morphologically to the endocrine status of a rodent [12, 13] and, hence, the submandibular salivary gland is suitable as a model organ in search of plausible lunaphasic morphological changes in the body.
The so-called light pinealocytes (LP) and dark pinealocytes (DP) are mosaically located in the pineal gland of the mouse. The cytoplasm of LP and DP contains vesicles that are presumably involved in the secretory activity of this endocrine organ as key participants of the lunaphasic response of the body. Based on this assumption, we searched and found morphological distinctions in some structures of pineal and submandibular salivary glands during the opposite phases of the lunar cycle. These distinctions are apparently the body’s response to the corresponding phases of the natural celestial satellite of the Earth.
MATERIALS AND METHODS
The study was performed in accordance with relevant institutional regulations on 12-week-old male mice (stock CD1) weighing 25 to 30 g (n = 22). The glands were removed at 11 a.m. in the different phases of the lunar cycle, after a 24-h food deprivation of animals. Salivary glands were fixed in 10% neutral formalin, then dehydrated and embedded in paraffin. The sections were stained with hematoxylin and eosin. The pineal glands were fixed by immersion in a mixture of 4% paraformaldehyde and 2.5% glutaraldehyde in a 0.1 M cacodylate buffer (pH 7.4), then post-fixed in a 1% solution of osmium tetroxide, dehydrated, and embedded in the Epon-Araldite resin mixture. The sections were prepared on a Leica EM UC 7 ultramicrotome (Austria). Semi-thin sections were stained with azure II. The light-microscopic study was performed on the Primo Star microscope (Carl Zeiss, Germany) with the G-10 digital camera (Canon, Japan) and Axio Vision 4.8.2 software (Carl Zeiss). The number of profiles of pinealocytes and large cytoplasmic vesicles with osmiophilic inclusions was counted in 10 fields of each section of the pineal gland having area of 0.011 mm2 (objective ×100, ocular ×10) followed by the measurement of their size, the area of nuclei, and the diameter of pinealocyte nucleoli. In a cross-section of the submandibular salivary glands, we measured the lumen area of the granular segments, the area of the nucleus, and the nuclear-cytoplasmic ratio of epitheliocytes. Ultra-thin sections of the pineal glands contrasted with uranyl acetate and lead citrate were examined on a Philips CM 12 electron microscope (Netherlands) with Megaview G 2 digital camera (Olympus, Germany). The data from the Excel 7.0 general matrix (Microsoft, USA) were processed by the methods of variation statistics using the Statistics for Windows v.6.0 software package. The arithmetic mean value of the index and the error of the mean were calculated. The significance of differences was judged by Student’s t-test. The differences were considered significant at P < 0.05.
RESULTS
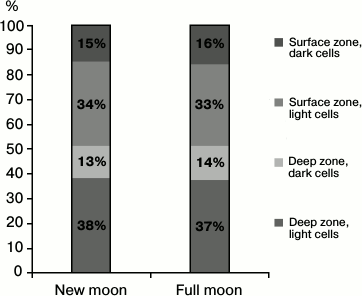
No significant differences between the total number of pinealocytes in the pineal gland of mice at the new and full moon were revealed. Also, the average area of the nucleus and the average diameter of the nucleolus of these cells were practically independent of the phases of the moon (table). The phases of the moon did not affect the distribution of the two types of pinealocytes, namely light (LP) and dark (DP) pinealocytes, among the zones of the organ, namely in the deep and surface zones of the pineal gland (Fig. 1).
Average number of pinealocytes and their karyometric indices for the
different phases of the moon (M ± m)
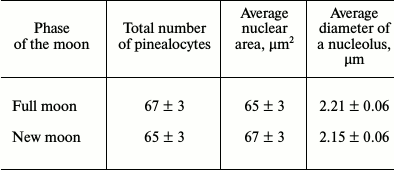
Fig. 1. Pinealocytes distribution by their types and by the zones of the pineal gland in the new moon and the full moon.
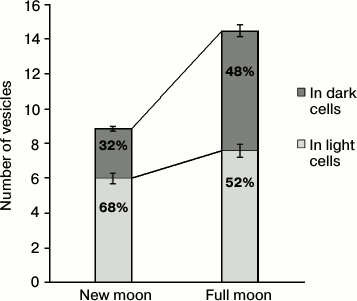
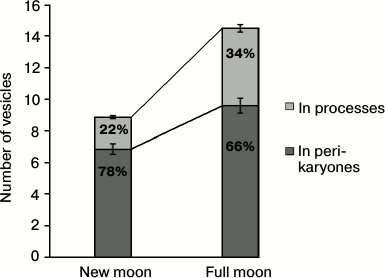
However, morphological differences for LP and DP between the new moon and the full moon proved to be significant at the intracellular level. Not only the total number of vesicles with osmiophilic inclusions increases (by more than half) in the full moon as compared with the new moon, but their distribution between different types of cells and within cells also proved to be uneven. Only one third of all vesicles were found in the DP in the new moon, whereas in the full moon there are almost half of them (Fig. 2). Vesicles with osmiophilic inclusions are basically found in perikaryons. Less than a quarter of all the vesicles are found in the processes of pinealocytes at the new moon, whereas at the full moon there are more than one third of them there, which indirectly indicates the shift of the vesicles, closer to the full moon, in the direction of the pinealocytic processes (Fig. 3).
Fig. 2. Distribution of vesicles with osmiophilic inclusions in light and dark cells at the different phases of the moon cycle. The proportion of vesicles of light and dark cells in the specified phase of the moon cycle is shown on the columns.
Fig. 3. Distribution of vesicles with osmiophilic inclusions in the processes and perikaryones of pinealocytes at the different phases of the moon cycle. The proportion of vesicles in the processes and perikaryones in the specified phase of the moon cycle is shown on the columns.
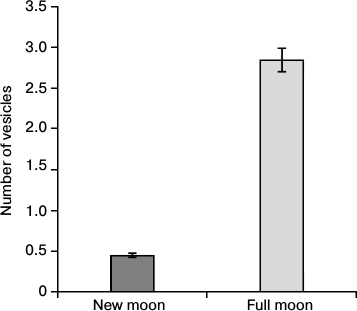
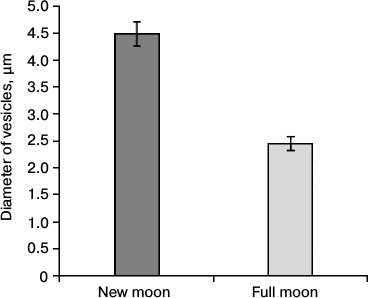
Figures 2-5 show large differences in the location and size of the vesicles with osmiophilic inclusions in the opposite phases of the lunar cycle, specifically, in the new moon and in the full moon. These differences can be seen when comparing the number of vesicles in the LP and DP in the surface and deep zones of the pineal gland, as well as when comparing representation of the vesicles in perikaryons and in the processes of pinealocytes. The number of vesicles in the processes of LP and DP of the deep zone of the gland increased 5.1 to 7.7 times in the full moon as compared with that in the new moon.
Fig. 4. Dependence of the number of vesicles in the pinealocytic processes within the deep zone of the pineal gland on the specified phase of the moon.
Fig. 5. Dependence of the diameter of the vesicles in the perikaryones of pinealocytes within the deep zone of the pineal gland on the specified phase of the moon.
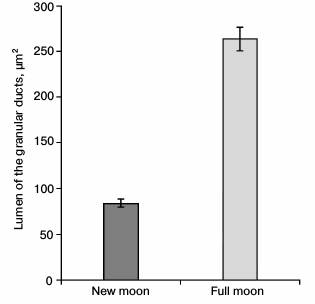
In the submandibular salivary glands of mice, the phasic changes of morphology are also associated with the lunar periodicity, and they were observed in the granular compartments of striated excretory ducts, which are involved in the secretory function. At the new moon as compared with the full moon, these salivary glands contain an increased number of large secretory granules and a reduced lumen area of granular compartments in cross section (Fig. 6); also in the new moon, the area of the nuclei in epitheliocytes and their nuclear-cytoplasmic ratio are reduced.
Fig. 6. Changes of the lumen of the granular ducts in the salivary gland during lunar phases.
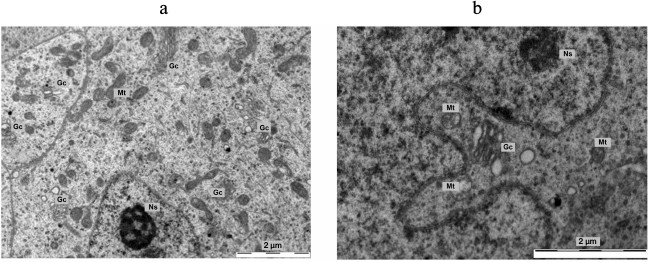
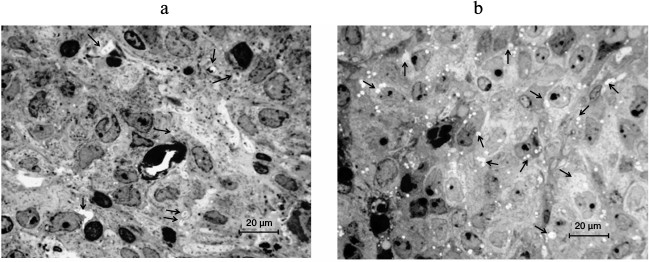
As concerns the origin of the vesicles, the following point should be noted. In the new moon, hyperplasia of dictyosomes and increase in the size of the Golgi apparatus area were observed in the LP with a large nucleus and nucleoli of the reticular type. In the full moon, dictyosomes are located near the nucleus, sometimes directly in the folds of karyolemma, dictyosomes break with the formation of vesicles, and the number of dictyosomes decreases. The photos shown in Fig. 7 depict the region of the Golgi complex in pinealocytes in the different phases of the moon. Vesicles represent themselves as containers, supposedly carrying in them not only dense inclusions, but also secretory factors (photos in Fig. 8).
Fig. 7. Photos of the Golgi complex in pinealocytes: a) hyperplasia of dictyosomes, expansion of the zone of their location, constrictions in mitochondria, a partial enlightenment of the mitochondrial matrix at the new moon; b) the Golgi complex in the folds of karyolemma, mitochondria with the signs of degradation at the full moon. Gc, Golgi complex; Mt, mitochondria; Ns, nucleolus.
Fig. 8. Photos of the vesicles with osmiophilic granules in pinealocytes (arrows) within the deep zone of the pineal gland in mice: a) in the new moon; b) in the full moon. Semi-fine sections. Azure II stain.
DISCUSSION
We have no evidence that changes in the submandibular salivary gland are caused by the primary changes in pinealocytes, but we believe that this is the case. Apparently, it is the gravitational influence of the Earth’s natural satellite that exerts an influence (without affecting the redistribution of cells in the tissues of the pineal gland) that is sufficient to induce morphological (and probably functional) changes in pinealocytes. These changes correlate with the phases of the moon. Since redistribution of the vesicles containing osmiophilic electron-dense inclusions, i.e. peculiar micrograins, and other morphological changes are in accordance with the phases of the moon, one can deduce that the cause of the observed changes is also based on the lunar influence. This is consistent with the prediction of the lunasensor hypothesis about the role of the pineal gland as a peculiar gravisensor [9, 10].
The ultrastructure of cytoplasmic vesicles with osmiophilic inclusions is variable in pinealocytes, but very characteristic. Vesicles are separated from cytosol by a single membrane and rich in an electron-transparent component, which probably contains water-soluble hormonal factors. As for electron-dense particles in murine pinealocytes, they are known as calcified material. Similar vesicular electron-dense concretions in rats contain considerably more osmiophilic material than in mice [6, 14, 15]. Taking into account the observed increase in the number of vesicles with osmiophilic inclusions occurring at the new moon (and at the background of hyperplasia of dictyosomes and an expanding area of the Golgi complex), one can speculate that the vesicles carrying dense inclusions are formed owing to the Golgi complex of pinealocytes. It is generally accepted that the functional state of the pineal endocrine cells – light pinealocytes (LP) and dark pinealocytes (DP) – is different [1, 2, 16]. The observed lunar phase-dependent morphological changes can be a reflection of the shifts in the functional state of the pineal gland. Under the influence of recurrent lunaphasic secretion of the pineal gland, different systems and organs of the body could also periodically change some of their properties, including submandibular gland as an example of such organs. Morphological criteria for evaluating the functional status of both the pineal gland and submandibular salivary glands are well known [1, 2, 6, 7, 13, 17-20], and they therefore can be used in search of the supposed primary processes responsible for the lunaphasic sensory system of a body. In the present study, the lunaphasic morphological changes were also observed, in addition to the pineal gland, in submandibular salivary gland. But we suppose that these salivary glands respond to the hormonal signals that were generated primarily by the pineal gland. Hormonal signals from pinealocytes could affect the hypothalamus and, through it, many other targets.
It was already reported about the moon-phase dependent activity of some functional systems of the body. According to intravital imaging data concerning the pineal gland in humans, its volume in the new moon significantly deviates from its mean value for a given age [21]. Epidemiological data indicate that the new moon is more dangerous for myocardial infarction than the full moon [22]. At the full moon, for example, the EEG delta activity in humans was reduced by 30% in the deeper stages of sleep, and evening melatonin secretion was also greatly suppressed [11]. Depending on the phase of the lunar cycle, human and animal behavior can change somewhat [23].
Thus, we have obtained the first evidence that the intrapinealocytic electron-dense concretions, or so to say micrograins, are actually redistributed in a manner coordinated with the phases of the moon. We suppose that regular changes in the gravitational influence of the moon are perceived by the cells of the pineal gland with the help of their vesicles, which simultaneously have both electron-dense osmiophilic micrograins and bioactive substances. Osmiophilic mineral concretions, after egesting into the intercellular space of a pineal gland and aggregating into larger clusters, could form there the so-called “brain sand”, which is known in this gland already for four centuries. Micrograins, still remaining within the vesicle of a cell, could begin to consolidate under the influence of the moon’s gravitational field into gradually growing aggregate. The growing micrograins could exert the mechanical effect on the adjacent cellular structures and thereby initiate the transportation of vesicles in the direction of pinealocytic clavate processes, in order to release the vesicular content into the extracellular space. Subsequently, the released hormones could flow through capillaries and liquor to the hypothalamus. Because of that, the neuroendocrine system could regulate, in a lunaphasic manner, the activity of different organs of the body, including, for example, the murine submandibular salivary gland. As an additional effect, the liquid constituent of vesicles can form so-called false vacuoles in the intercellular space of the pineal gland. Such kind of vacuoles, though without any connection with the role of the moon, was long ago described for the gerbil pineal gland [4].
The basic morphological effect described here – a lunaphasic mode of redistribution of pinealocytic vesicles with electron-dense concretions (i.e. micrograins surrounded by the secretory material) – can have far reaching consequences for gerontology. If the revealed effect is really used by the endocrine-gravisensory pacemaker of the lifelong clock that participates in development, maturation, and aging, then an artificial, though harmless, shutdown of this pacemaker could become the most radical procedure to repeal aging.
The interval between the point, when the physiological maturation has been completed, and the point of completion of the life itself, has no special name. It is to this interval one can apply the term phenoptosis [24], understanding it somewhat anew, namely as a period of an age-dependent loss of some characters of an aged organism. Just at this time, many of them, mainly quantitative traits (strength of muscles, acuity of vision, hearing and olfactory, as well as the loss of proper hormonal balance, etc.) begin to “fade”, weakening the body literally on all fronts. The duration of such phenoptotic period varies in different species. It seems most expedient to stop the signals from the endocrine-gravitational pacemaker of a pineal gland at the beginning of this period, and this will be done as soon as such procedure will become practically attainable.
This study was supported in part by a grant from the Rostock Group (CEO A. V. Chikunov).
REFERENCES
1.Karasek, M. (1992) Ultrastructure of the mammalian
pinealocyte under natural and experimental conditions: quantitative
aspects, Microsc. Res. Tech., 21, 116-123.
2.Karasek, M., and Reiter, R. J. (1992)
Morphofunctional aspects of the mammalian pineal gland, Microsc.
Res. Tech., 21, 136-157.
3.Karasek, M., Zielinska, A., Marek, K., and
Swietoslawski, J. (2002) Effect of superior cervical ganglioectomy on
the ultrastructure of pinealocytes in the Djungarian hamster
(Phodopus sungorus): quantitative study, Neuroendocrinol.
Lett., 23, 443-446.
4.Reiter, R. J. (1981) Chronobiological aspects of
the mammalian pineal gland, Prog. Clin. Biol. Res., 59C,
223-233.
5.Sabry, I., Al-Ghaith, L., and Al-Azemi, M. (1999)
Pineal gland of the Kuwaiti desert gerbil (Gerbillus cheesmani):
alterations of its structure by bromocriptine treatment, Endocr.
Regul., 33, 69-78.
6.Logvinov, S. V., Gerasimov, A. V., and
Kostyuchenko, V. P. (2004) Ultrastructure of pinealocytes in rats
exposed to light and radiation, Morphology, 125,
71-75.
7.Gerasimov, A. V., Logvinov, S. V., Kostyuchenko, V.
P., and Kravchenko, L. B. (2012) Morphology of the pineal gland of mice
with delayed puberty, Byul. Sib. Med., 10, 22-25.
8.Lewczuk, B., Nowicki, M., Prusik, M., and
Przybylska-Gornowicz, B. (2004) Diurnal rhythms of pinealocyte
ultrastructure, pineal serotonin content and plasma melatonin level in
the domestic pig, Folia Histochem. Cytobiol., 42,
155-163.
9.Olovnikov, A. (2005) Lunasensor, infradian rhythms,
telomeres, and the chronomere program of aging, Ann. N. Y. Acad.
Sci., 1057, 112-132.
10.Olovnikov, A. M. (2007) Hypothesis: Lifespan is
regulated by chronomere DNA of the hypothalamus, J.
Alzheimer’s Dis., 11, 241-252.
11.Cajochen, C., Altanay-Ekici, S., Munch, M., Frey,
S., Knoblauch, V., and Wirz-Justice, A. (2013) Evidence that the lunar
cycle influences human sleep, Curr. Biol., 23,
1485-1488.
12.Jayasinghe, N. R., Cope, G. H., and Jacob, S.
(1990) Morphometric studies on the development and sexual dimorphism of
the submandibular gland of the mouse, J. Anat., 172,
115-127.
13.Logvinov, S. V., and Gerasimov, A. V. (2007)
Circadian System and Adaptation. Morphofunctional and
Radiobiological Aspects [in Russian], Pechatnaya Manufaktura,
Tomsk, p. 200.
14.Pongsa-Asawapaiboon, A., Asavaritikrai, P.,
Withyachumnarnkul, B., and Sumridthong, A. (1998) Melatonin increases
nerve growth factor in mouse submandibular gland, J. Pineal
Res., 24, 73-77.
15.Allen, D. J., DiDio, L. J. A., and Gentry, E. R.
(1982) The aged rat pineal gland as revealed in SEM and TEM,
Age, 5, 119-126.
16.Vollrath, L. (1984) Functional anatomy of the
human pineal gland, in The Pineal Gland (Reiter, R. J., ed.)
Raven Press, N. Y., pp. 285-322.
17.Gerasimov, A. V., Logvinov, S. V., and
Kostyuchenko, V. P. (2010) Morphological changes in the pineal gland of
rats after a prolonged exposure to illumination with bright light,
Bull. Exp. Biol. Med., 150, 97-99.
18.Denny, P. C., Chai, Y., Pimprapaiporn, W., and
Denny, P. A. (1990) Three-dimensional reconstruction of adult female
mouse submandibular gland secretory structures, Anat. Rec.,
226, 489-500.
19.Tandler, B., Gresik, E. W., Nagato, T., and
Phillips, C. J. (2001) Secretion by striated ducts of mammalian major
salivary glands: review from an ultrastructural, functional, and
evolutionary perspective, Anat. Rec., 264, 121-145.
20.Logvinov, S. V., Gerasimov, A. V., and
Kostyuchenko, V. P. (2003) Structural changes in epitheliocytes of
granular excretory ducts and terminal sections of submandibular glands
of rats after exposure to light and radiation, Morphology,
124, 80-83.
21.Ivanov, S. V. (2008) Substrates and possible
mechanisms of pineal gland’s moon-sensory function in the context
of redusome hypothesis of aging and control of biological time in
ontogeny, Adv. Gerontol., 21, 488-490.
22.Ivanov S. V., and Kostoglodov, Iu. K. (2010)
Morphological and chronoepidemiological motivation of the pineal
gland’s lunasensory function in the context of redumere
hypothesis of aging, Adv. Gerontol., 23, 536-538.
23.Zimecki, M. (2006) The lunar cycle: effects on
human and animal behavior and physiology, Postepy Hig. Med.
Dosw., 60, 1-7.
24.Skulachev, V. P., Skulachev, M. V., and Feniouk,
B. A. (2013) Life with no Aging [in Russian], Eksmo, Moscow.