Therapeutic Doses of SkQ1 Do Not Induce Cytochromes P450 in Rat Liver
K. N. Myasoedova1,2* and D. N. Silachev2,3
1Lomonosov Moscow State University, Faculty of Fundamental Medicine, 119991 Moscow, Russia; fax: (499) 726-5547; E-mail: skulach@belozersky.msu.ru; kseniamyasoedova@gmail.com2Lomonosov Moscow State University, Institute of Mitoengineering, 119991 Moscow, Russia; E-mail: proteins@mail.ru
3Lomonosov Moscow State University, Belozersky Institute of Physico-Chemical Biology, 119991 Moscow, Russia
* To whom correspondence should be addressed.
Received June 20, 2014
The effect of SkQ1 (a mitochondria-targeted antioxidant) on the level of cytochromes P450 in rat liver was studied. It was found that administration of therapeutic dose of SkQ1 with drinking water for 5 days (250 nmol/kg of body weight per day) did not alter the level of cytochromes P450. Under the same conditions, the standard dose of phenobarbital used for the induction of cytochromes P450 caused the 2.7-fold increase in the content of these cytochromes. We conclude that therapeutic doses of SkQ1 do not induce cytochromes P450 in rats.
KEY WORDS: cytochrome P450, mitochondria-targeted antioxidant, SkQ1DOI: 10.1134/S0006297914100149
Abbreviations: SkQ1, plastoquinonyl-decyltriphenylphosphonium.
Many xenobiotics, when ingested by mammals, can cause an increase in the
content of autooxidizable hemoproteins in their liver cells. The
hemoproteins are known as cytochromes P450 because of their specific
absorption band at 450 nm in the spectrum after reduction with
with carbon oxide. Xenobiotics undergo oxidative transformations in the
animal’s liver, these processes being catalyzed by monooxygenase
enzyme systems of endoplasmic reticulum including NADPH-specific
flavoprotein and cytochromes P450. Biotransformation of xenobiotics is
primarily a function of liver cytochromes P450. In other tissues
cytochromes of this group (57 genes coding for cytochromes P450 have
been found so far in the human genome) mainly carry out various
reactions of endogenous substrates, including reactions of biosynthesis
and metabolism of a number of physiologically active compounds [1-6]. Already during the first
decade following the discovery of cytochrome P450 in 1958, over 200
chemical compounds of various structure (pharmaceuticals, narcotic
drugs, toxins, various factors of environmental pollution, etc.)
capable of inducing a reversible increase in the content of cytochromes
P450 in liver cells have been described [5]. This
list is continually supplemented. One of the most studied forms of
cytochrome P450 from human liver (3A4) can metabolize up to 50% of all
drugs available on the pharmacological market [3,
4, 6].
The extremely broad substrate specificity is related to the unusual features of the catalytic effect of cytochromes P450, a phenomenon that still has no clear explanation.
It should be noted that until recently liver cytochromes P450 were considered components of the organism’s main detoxification system, which oxidizes and thereby makes more hydrophilic alien compounds, facilitating their excretion. However, it has been shown that sometimes oxidation products are more dangerous (toxic, carcinogenic, etc.) for the organism than the original compounds. Specifically, activation of potential carcinogens, e.g. polycyclic aromatic hydrocarbons (in particular, benzo(a)pyrene), is an example [2, 6]. This makes it necessary to study the possible induction of liver cytochromes P450 by new medical drugs recommended for the treatment of various pathologies.
The synthetic derivative of plastoquinone conjugated with the penetrating cation decyltriphenylphosphonium (SkQ1) is one such drug. This compound was synthesized in our laboratory in 2008 [7], passed preclinical and clinical trials [7-12], was recommended for use in the treatment of a number of human diseases, and is on the market since 2012. In 2008, N. G. Kolosova et al. studied the possible effects of SkQ1 (50 or 250 nmol/kg of body weight per day administered with food for 1.5 months) on the activity of five isoforms of cytochrome P450 in rat liver (SkQ1 therapeutic doses in preclinical trials carried out on rats were 50-250 nmol/kg). The authors measured the activities of isoforms 1A2, 2B + 2C, and 2B1. Their experiments showed no reliable changes that could be indicative of the SkQ1-mediated induction of any of these isoforms [11]. However, there remained the possibility of the induction of some other cytochrome isoform.
In this study, we measured the total amount of cytochromes P450 in the liver of: 1) control rats; 2) rats receiving 250 nmol SkQ1/kg per day in drinking water for 5 days; 3) rats receiving 0.1% aqueous solution of phenobarbital instead of drinking water for the same time period (phenobarbital is a classical inducer of cytochrome P450 commonly used as a positive control in the studies of new pharmacological drugs).
The experiments were conducted with outbred male albino rats weighing 200 ± 10 g receiving regular laboratory feed; SkQ1 solution (250 nmol/kg of body weight per day) was given instead of drinking water for 5 days. Rats from the control group received regular drinking water. The third group of animals was used to evaluate their ability to react to administration of xenobiotics inducing cytochromes P450 in liver. These rats received 0.1% solution of sodium phenobarbital instead of drinking water for five days. Before being euthanized, the rats were deprived of food for one day. The animals were decapitated, then the liver was excised and thoroughly perfused with cold physiological saline, blended in a small volume of 0.15 M KCl and 5 mM EDTA (4oC), and homogenized in the same solution at a ratio 1 : 3 in a Dounce homogenizer with a Teflon pestle. Microsomal fraction was obtained by the conventional method of differential centrifugation. The final pellet obtained after centrifugation for 1 h at 105,000g was carefully washed with buffer containing 100 mM KH2PO4, 100 mM KCl, 1 mM EDTA, 1 mM dithiothreitol, and 20% glycerol, pH 7.4. The precipitate was resuspended in a small volume of the same buffer. The total amount of cytochromes P450 in the microsomes was determined spectrophotometrically by the method of Omura and Sato [13] using the absorption by the dithionite-reduced complex of cytochromes P450 with carbon monoxide at 450 nm using molar extinction coefficient 91 mM–1·cm–1. Protein content was determined by the Lowry method [14].
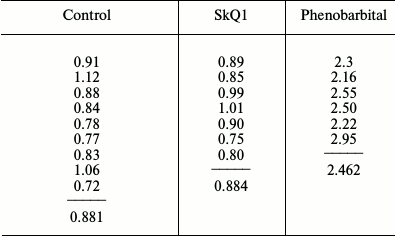
The results of the experiments are given in the table, which indicates the content of cytochromes P450 in the liver of each of 22 rats and the mean values for the three groups of animals. It can be seen that the data of the control group receiving neither SkQ1 nor phenobarbital fully coincided with those of the SkQ1-treated group. At the same time, phenobarbital within expected limits induced the synthesis of cytochromes P450, the amount of which increased 2.5-fold versus rats that received no phenobarbital. Thus, our data on the measurement of the total amount of all forms of cytochromes P450 in rat liver confirmed the data by Kolosova et al., who measured the activity of five isoforms of this cytochrome [11], namely confirming the lack of induction of cytochrome P450 by a therapeutic dose of SkQ1. Apparently, either SkQ1 is not capable of inducing these cytochromes or the therapeutic dose of SkQ1 (250 nmol/kg of body weight per day) is too small to cause such an effect, which might be dangerous from toxicological or carcinogenic perspectives.
Comparison of the effects of SkQ1 and phenobarbital on the content of
cytochromes P450 in rat liver microsomes (the amount of cytochromes
P450 is given in nmol per mg of microsomal protein)
REFERENCES
1.Furge, L. L., and Guengerich, F. P. (2006)
Cytochrome P450 enzymes in drug metabolism and chemical toxicology: an
introduction, Biochem. Mol. Biol. Ed., 34, 66-74.
2.Coon, M. J. (2002) Enzyme ingenuity in biological
oxidations: a trail leading to cytochrome P450, J. Biol. Chem.,
277, 28351-28363.
3.Ekroos, M., and Sjogren, T. (2006) Structural basis
for ligand promiscuity in cytochrome P450 3A4, Proc. Natl. Acad.
Sci. USA, 103, 13682-13687.
4.Guengerich, F. P. (2006) A malleable catalyst
dominates the metabolism of drugs, Proc. Natl. Acad. Sci. USA,
103, 13565-13566.
5.Lyakhovich, V. V., and Tsyrlov, I. B. (1981)
Induction of Xenobiotic Metabolism Enzymes [in Russian], Nauka,
Novosibirsk.
6.Myasoedova, K. N. (2008) New findings in studies of
cytochromes P450, Biochemistry (Moscow), 73, 965-969.
7.Antonenko, Y. N., Avetisyan, A. V., Bakeeva, L. E.,
Chernyak, B. V., Chertkov, V. A., Domnina, L. V., Ivanova, O. Yu.,
Izyumov, D. S., Khailova, L. S., Klishin, S. S., Korshunova, G. A.,
Lyamzaev, K. G., Muntyan, M. S., Nepryakhina, O. K., Pashkovskaya, A.
A., Pletjushkina, O. Yu., Pustovidko, A. V., Roginsky, V. A.,
Rokitskaya, T. I., Ruuge, E. K., Saprunova, V. B., Severina, I. I.,
Simonyan, R. A., Skulachev, I. V., Skulachev, M. V., Sumbatyan, N. V.,
Sviryaeva, I. V., Tashlitsky, V. N., Vassiliev, J. M., Vyssokikh, M.
Yu., Yaguzhinsky, L. S., Zamyatin, A. A., Jr., and Skulachev, V. P.
(2008) Mitochondria-targeted plastoquinone derivatives as tools to
interrupt execution of the aging program. I. Cationic plastoquinone
derivatives: synthesis and in vitro studies, Biochemistry
(Moscow), 73, 1273-1287.
8.Skulachev, V. P., Anisimov, V. N., Antonenko, Y.
N., Bakeeva, L. E., Chernyak, B. V., Erichev, V. P., Filenko, O. F.,
Kalinina, N. I., Kapelko, V. I., Kolosova, N. G., Kopnin, B. P.,
Korshunova, G. A., Lichinitser, M. R., Obukhova, L. A., Pasyukova, E.
G., Pisarenko, O. I., Roginsky, V. A., Ruuge, E. K., Senin, I. I.,
Severina, I. I., Skulachev, M. V., Spivak, I. M., Tashlitsky, V. N.,
Tkachuk, V. A., Vyssokikh, M. Y., Yaguzhinsky, L. S., and Zorov, D. B.
(2009) An attempt to prevent senescence: a mitochondrial approach,
Biochim. Biophys. Acta, 1787, 437-461.
9.Severin, F. F., Severina, I. I., Antonenko, Y. N.,
Rokitskaya, T. I., Cherepanov, D. A., Mokhova, E. N., Vyssokikh, M. Y.,
Pustovidko, A. V., Markova, O. V., Yaguzhinsky, L. S., Korshunova, G.
A., Sumbatyan, N. V., Skulachev, M. V., and Skulachev, V. P. (2010)
Penetrating cation/fatty acid anion pair as a mitochondria-targeted
protonophore, Proc. Natl. Acad. Sci. USA, 107,
663-668.
10.Skulachev, M. V., Antonenko, Y. N., Anisimov, V.
N., Chernyak, B. V., Cherepanov, D. A., Chistyakov, V. A., Egorov, M.
V., Kolosova, N. G., Korshunova, G. A., Lyamzaev, K. G., Plotnikov, E.
Y., Roginsky, V. A., Savchenko, A. Y., Severina, I. I., Severin, F. F.,
Shkurat, T. P., Tashlitsky, V. N., Shidlovsky, K. M., Vyssokikh, M. Y.,
Zamyatnin, A. A., Jr., Zorov, D. B., and Skulachev, V. P. (2011)
Mitochondrial-targeted plastoquinone derivatives. Effect on senescence
and acute age-related pathologies, Curr. Drug Targets,
12, 800-826.
11.Neroev, V. P., Archipova, M. M., Bakeeva, L. E.,
Fursova, A. Zh., Grigorian, E. N., Grishanova, A. Yu., Iomdina, E. N.,
Ivashchenko, Zh. N., Katargina, L. A., Khoroshilova-Maslova, I. P.,
Kilina, O. V., Kolosova, N. G., Kopenkin, E. P., Korshunov, S. S.,
Kovaleva, N. A., Novikova, Yu. P., Philippov, P. P., Pilipenko, D. I.,
Robustova, O. V., Saprunova, V. B., Senin, I. I., Skulachev, M. V.,
Sotnikova, I. F., Stefanova, N. A., Tikhomirova, N. K., Tsapenko, I.
V., Shchipanova, A. I., Zinovkin, R. A., and Skulachev, V. P. (2008)
Mitochondria-targeted plastoquinone derivatives as tools to interrupt
execution of the aging program. 4. Age-related eye disease. SkQ1
returns vision to blind animals, Biochemistry (Moscow),
73, 1317-1328.
12.Yani, Ye. V., Katargina, L. A., Chesnokova, N.
B., Beznos, O. V., Savchenko, A. Yu., Vygodin, Ye. Yu., Gudkova, Ye.
Yu., Zamyatnin, A. A., Jr., and Skulachev, M. V. (2012) The first
experience of using Visomitin in the treatment of the “dry
eye”, Prakt. Med., 1, 134-137.
13.Omura, T., and Sato, R. (1964) Carbon
monoxide-binding pigment of liver microsomes. I. Evidence for its
hemoprotein nature, J. Biol. Chem., 239, 2370-2378.
14.Dawson, R., Elliott, D., and Jones, K. (1991)
Directory of Biochemist [Russian translation], Mir, Moscow.